1. Halliwell R. Revised nomenclature for veterinary allergy. Vet Immunol Immunopathol 2006;114:207-208.


2. Bizikova P, Pucheu-Haston CM, Eisenschenk MN, Marsella R, Nuttall T, Santoro D. Review: Role of genetics and the environment in the pathogenesis of canine atopic dermatitis. Vet Dermatol 2015;26:95-e26.


3. Hillier A, Griffin CE. The ACVD task force on canine atopic dermatitis (I): incidence and prevalence. Vet Immunol Immunopathol 2001;81:147-151.


5. Marsella R. Evaluation of
Lactobacillus rhamnosus strain GG for the prevention of atopic dermatitis in dogs. Am J Vet Res 2009;70:735-740.


6. Marsella R, Santoro D, Ahrens K. Early exposure to probiotics in a canine model of atopic dermatitis has long-term clinical and immunological effects. Vet Immunol Immunopathol 2012;146:185-189.


7. Ohshima-Terada Y, Higuchi Y, Kumagai T, Hagihara A, Nagata M. Complementary effect of oral administration of
Lactobacillus paracasei K71 on canine atopic dermatitis. Vet Dermatol 2015;26:350-353.


8. Rosenfeldt V, Benfeldt E, Nielsen SD, Michaelsen KF, Jeppesen DL, Valerius NH, Paerregaard A. Effect of probiotic
Lactobacillus strains in children with atopic dermatitis. J Allergy Clin Immunol 2003;111:389-395.


10. Yoshida Y, Seki T, Matsunaka H, Watanabe T, Shindo M, Yamada N, Yamamoto O. Clinical effects of probiotic Bifidobacterium breve supplementation in adult patients with atopic dermatitis. Yonago Acta Med 2010;53:37-45.
12. Björkstén B, Sepp E, Julge K, Voor T, Mikelsaar M. Allergy development and the intestinal microflora during the first year of life. J Allergy Clin Immunol 2001;108:516-520.


13. Yamamoto K, Yokoyama K, Matsukawa T, Kato S, Kato S, Yamada K, Hirota T. Efficacy of prolonged ingestion of
Lactobacillus acidophilus L-92 in adult patients with atopic dermatitis. J Dairy Sci 2016;99:5039-5046.


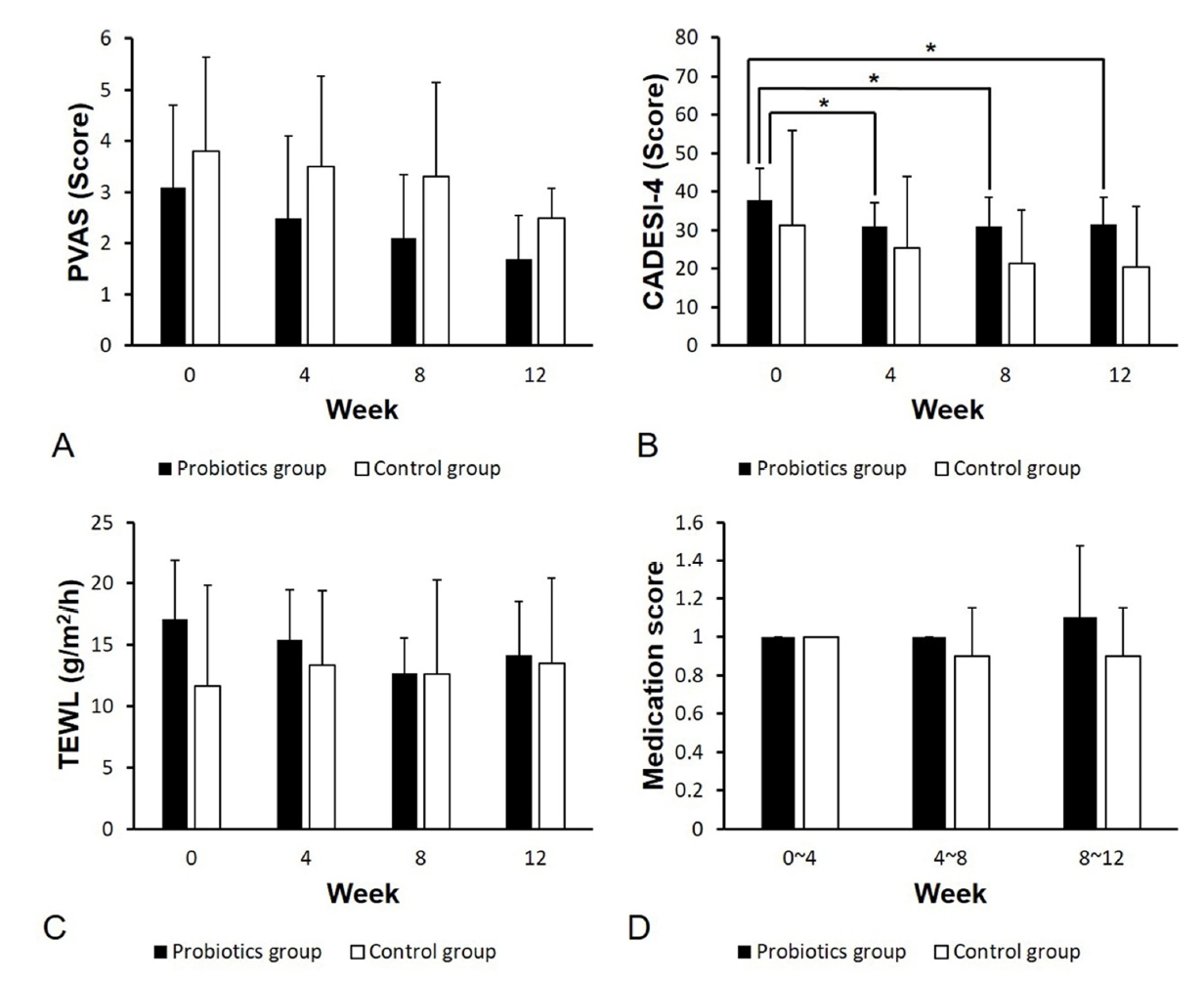
15. Olivry T, Saridomichelakis M, Nuttall T, Bensignor E, Griffin CE, Hill PB; International Committe on Allergic Diseases of Animals (ICADA). Validation of the Canine Atopic Dermatitis Extent and Severity Index (CADESI)-4, a simplified severity scale for assessing skin lesions of atopic dermatitis in dogs. Vet Dermatol 2014;25:77-85.


16. Rybnícek J, Lau-Gillard PJ, Harvey R, Hill PB. Further validation of a pruritus severity scale for use in dogs. Vet Dermatol 2009;20:115-122.


17. Huurre A, Laitinen K, Rautava S, Korkeamäki M, Isolauri E. Impact of maternal atopy and probiotic supplementation during pregnancy on infant sensitization: a double-blind placebo-controlled study. Clin Exp Allergy 2008;38:1342-1348.


18. Kim HJ, Kim YJ, Kang MJ, Seo JH, Kim HY, Jeong SK, Lee SH, Kim JM, Hong SJ. A novel mouse model of atopic dermatitis with epicutaneous allergen sensitization and the effect of
Lactobacillus rhamnosus. Exp Dermatol 2012;21:672-675.


20. Kim HJ, Kim YJ, Lee SH, Yu J, Jeong SK, Hong SJ. Effects of
Lactobacillus rhamnosus on allergic march model by suppressing Th2, Th17, and TSLP responses via CD4
+ CD25
+ Foxp
3+ Tregs. Clin Immunol 2014;153:178-186.


21. Larsen N, Vogensen FK, Gøbel R, Michaelsen KF, Abu Al-Soud W, Sørensen SJ, Hansen LH, Jakobsen M. Predominant genera of fecal microbiota in children with atopic dermatitis are not altered by intake of probiotic bacteria
Lactobacillus acidophilus NCFM and
Bifidobacterium animalis subsp.
lactis Bi-07. FEMS Microbiol Ecol 2011;75:482-496.


22. Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013;504:446-450.


23. Purchiaroni F, Tortora A, Gabrielli M, Bertucci F, Gigante G, Ianiro G, Ojetti V, Scarpellini E, Gasbarrini A. The role of intestinal microbiota and the immune system. Eur Rev Med Pharmacol Sci 2013;17:323-333.

24. Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev 2010;90:859-904.


25. Suchodolski JS, Simpson K. Canine gastrointestinal microbiome in health and disease. Veterinary Focus 2013;23:22-28.
28. Brouwer ML, Wolt-Plompen SA, Dubois AE, van der Heide S, Jansen DF, Hoijer MA, Kauffman HF, Duiverman EJ. No effects of probiotics on atopic dermatitis in infancy: a randomized placebo-controlled trial. Clin Exp Allergy 2006;36:899-906.


29. Kopp MV, Hennemuth I, Heinzmann A, Urbanek R. Randomized, double-blind, placebo-controlled trial of probiotics for primary prevention: no clinical effects of
Lactobacillus GG supplementation. Pediatrics 2008;121:e850-e856.


30. Doron S, Snydman DR. Risk and safety of probiotics. Clin Infect Dis 2015;60 Suppl 2:S129-S134.














 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print