The third eyelid gland lies at the base of the nictitating membrane and surrounds the base of the vertical cartilage of the third eyelid [
1]. Although the overall frequency of the third eyelid gland tumors is low, the majority of tumors originating from the third eyelid gland are adenocarcinomas [
2]. The tumor location is important to differentiate the third eyelid gland origin mass from other orbital masses [
3-
5]. Computed tomography (CT) examination allows to determine the exact location of the mass and understand the anatomic relationship between the ipsilateral globe and adjacent structures. CT also delineates the margin of the mass and evaluates bone lesions and calcification of the soft tissue with excellent contrast resolution [
3,
4]. CT findings of the third eyelid gland adenocarcinoma have been reported only in two dogs [
3,
6]. The third eyelid gland mass invaded the retrobulbar space and induced exophthalmos in both dogs [
3,
6]. In one dog, the focal invasion of the tumor into the posterior wall of the globe was observed on CT images [
3]. Although the invasion has not been reported in the previous two cases, the third eyelid gland tumor can invade the nasolacrimal drainage apparatus due to the infiltrative character of the adenocarcinomas. Therefore, in this report, we described the CT findings of the third eyelid gland adenocarcinoma about the location, enhancement pattern, secondary exophthalmos, and the lysis of the body nasolacrimal duct which is not observed in the previous cases.
A 15-year-old castrated male Schnauzer was presented with right exophthalmos. Ophthalmic examination showed a pink, firm mass located ventral to the right globe with mild protrusion of the third eyelid. There were no abnormal findings in the right eye globe except for exophthalmos due to the mass. Blood test results did not reveal any specific findings. Ultrasonography revealed a mass with an anechoic center caudal to the right globe. However, the exact location, size, extent, and origin of the mass could not be determined due to poor acoustic window by the orbit.
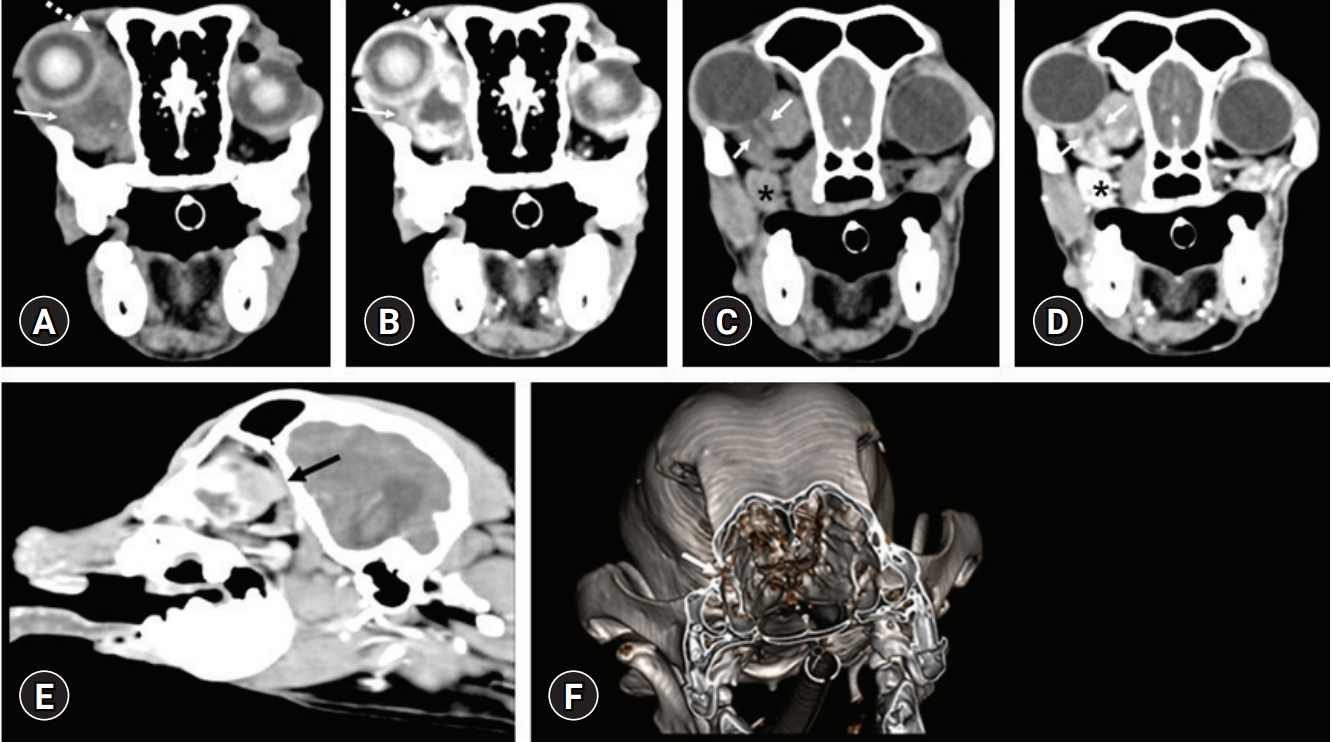
CT images were acquired using a multi-detector CT scanner (Emotion 16; Siemens, Germany) with the following settings: 110 kVp, 130 mA; slice thickness, 1 mm; and pitch, 1. Post-contrast CT images were obtained after intravenous injection of 640 mg iodine/kg ioversol (Optiray; Liebel-Flarsheim, USA) using a power injector (Mallinckrodt; Liebel-Flarsheim). On pre-contrast CT images, a mass of 20 × 21 × 29 mm in size was located ventromedial to the right globe (
Fig. 1A-
E). The mass with a few mineral density foci was hyperattenuating (63 HU) compared to the retrobulbar fat and had a clear margin from the fat. There were multiple hypoattenuating regions (16-32 HU) within the mass. The largest region of the hypoattenuating region was 13 × 10 × 9 mm in size and located ventrocaudal to the globe. The mass was enhanced moderately except for the hypoattenuating center. A tubular structure with 2 mm diameter was shown as double hypoattenuating parallel lines in the mass at the level of the frontal sinus and coursed medially (
Fig. 1C and
D). The right main lacrimal gland and zygomatic gland were observed normally. However, the right third eyelid gland was not identified separately. The mass at the level of the right third eyelid gland occupied from the rostral of the globe to the retrobulbar region and caused marked dorsolateral displacement of the right globe (
Fig. 1E). Mild erosive change of the bony nasolacrimal duct was observed (
Fig. 1F). There were no lytic or proliferative changes in the surrounding bones, including the frontal, lacrimal, maxillary, zygomatic, palatine, and sphenoid bones.
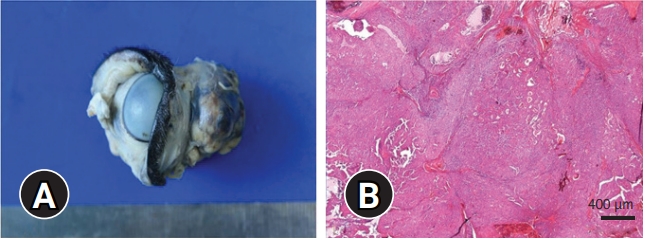
Enucleation and surgical excision of the third eyelid gland mass were performed for therapeutic and diagnostic purposes. Grossly, the mass was irregular in shape with a diameter around 25 × 15 mm (
Fig. 2A). The cut surface of the mass appeared white at the center and black-brown in the periphery. On histological examination, papillary, tubular, and solid nested patterns of lesions were observed (
Fig. 2B). The mass did not show any atypia, including the high cellular pleomorphism and mitotic figures; however, it invaded into the retrobulbar space indicating its malignancy. The positive results on Periodic-Schiff stain and Alcian blue stain indicated that this tumor was of the lacrimal serous gland origin and excluded the mucin producing zygomatic gland origin. Immunohistochemistry revealed that the tumor was strongly positive for cytokeratin and partially positive for the proliferating cell nuclear antigen but was negative for vimentin. Finally, the mass was diagnosed as adenocarcinoma originating from the third eyelid gland using histological examination and immunohistochemistry.
After surgery, the dog was recovered from anesthesia uneventfully and restored without any swelling or inflammation of the surgical region. However, unfortunately, the dog was lost to follow-up.
In this study, a third eyelid gland tumor was diagnosed based on the enhancement and location of the mass, direction of the secondary displacement of the ipsilateral globe, and absence of normal third eyelid gland through CT images and confirmed as adenocarcinoma on histologic examination. Although thinning of the adjacent bones meaning possibility of invasion was observed in the previous case [
6], there was no change of the orbital bones in our case. Instead, the tumor invasion into the bony nasolacrimal duct was observed on CT images. This finding can be used as a sign of aggressive nature and a potential complication of the neoplastic process.
Diagnostic imaging is needed to localize the mass to the third eyelid gland and differentiate it from the mass originating from adjacent tissues [
1,
3,
7]. The orbital masses can be originated primarily from the muscle of the eye, fat, nerves, blood vessels, and tear glands. In addition, secondary tumors arising from the adjacent structures such as the salivary gland, nasal cavity, or oral cavity can invade into the orbital region. Ocular ultrasound is effective for the visualization of the intraocular and retrobulbar structures. However, the orbit is the bony socket. Therefore, ultrasonography is difficult to access the lesion to visualize the orbital masses [
8].
CT images can delineate the size, extent, location, and anatomic relation with adjacent structures of the third eyelid gland mass. In particular, the displacement of the adjacent structures, including the globe by the mass, can help to localize the third eyelid gland mass. In the present case, the third eyelid gland adenocarcinoma invaded the retrobulbar space through the ventromedial aspect of the ipsilateral globe and displaced the globe dorsolaterally, like the previous two cases [
3,
6]. Another dog with basal cell carcinoma originating from the third eyelid gland showed dorsal displacement of the ipsilateral eye globe [
7]. However, exophthalmos can be caused by primary orbital conditions such as abscess, cellulitis, and tumors, or by disease extension from adjacent structures such as zygomatic sialadenitis, zygomatic salivary mucocele, and masticatory myositis [
9]. Therefore, it is difficult to diagnose the third eyelid gland tumor based on exophthalmos.
Although often challenging, the mass originated from the third eyelid gland can be differentiated from other orbital masses based on the tumor location on CT images [
3,
5]. The mass arising from the main lacrimal gland tumor should be included in the differential list of the third eyelid gland tumor because both can show similar appearances on histologic examination [
10,
11].
However, the main lacrimal gland is located on the superotemporal surface of the globe; hence, it can displace the ipsilateral eyeball ventromedially [
1,
12]. Meanwhile, the zygomatic salivary gland is located ventral to the eye globe. The lesions originating from the zygomatic salivary gland can cause dorsal displacement of the eye globe, similar to the third eyelid gland tumor [
13]. Therefore, the mass originating from the zygomatic salivary gland was included in the differential list in this case. In the present case, the ipsilateral zygomatic salivary gland was typically identified separately from the mass in this case. Therefore, the mass originating from the zygomatic salivary gland could be ruled out.
The invasion of the third eyelid gland adenocarcinomas into the posterior wall of the ipsilateral eye was identified in a dog of the previous report [
3]. In our case, the invasion of the third eyelid gland adenocarcinoma into the nasolacrimal drainage apparatus was found as the mild osteolysis of the bony nasolacrimal duct. The bony nasolacrimal duct is the component of the nasolacrimal drainage apparatus, a route for releasing tears produced by the third eyelid gland [
14]. In addition, the hypoattenuating tubular structure in the mass was observed, which may be the dilated canaliculus; however, unfortunately, this finding was not confirmed through histologic examination or CT dacryocystography [
15].
In conclusion, the third eyelid gland adenocarcinoma appeared as a heterogeneous cystic mass and moderately enhanced. The mass caused exophthalmos dorsolaterally. CT can determine the location of the mass in the retrobulbar space, and loss of normal third eyelid gland appearance helped determine the mass origin. In addition, osteolysis of the bony nasolacrimal duct showed the invasive character of the adenocarcinoma. These findings could help distinguish the third eyelid gland adenocarcinoma from other orbital diseases.










 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



