Bovine anemia due to
Theileria orientalis group (BATOG) is a tick-borne disease (TBD) affecting cattle, with
Haemaphysalis spp. as the major tick involved in disease transmission [
1]. Unlike the lymphoproliferative
Theileria species, BATOG mainly affects the erythrocytes (RBC) and RBC-associated parameters [
2]. Clinical signs include weakness, abortion, fever, pallor, and elevated respiratory and heart rates [
2]. However, few clinical cases of
T. orientalis infection in cattle have been reported. Consequently,
T. orientalis was historically considered to cause benign infection only [
2]. Despite the minimal pathogenic effects of BATOG to the cattle, significant losses have been associated due to increased treatment costs, and reduced body weight, production, and reproductive performance, and ultimately, poor welfare and death [
2]. BATOG is also known as ‘oriental theileriosis’ because the main vector, Asian long-horned tick, was previously thought to be endemic in East-Asian countries only. But in the past decade, outbreaks of BATOG have been reported in many countries, including several Asian and Asia-Pacific countries [
2], and recently the United States [
3].
T. orientalis is categorized onto 11 genotypes, with Chitose and Ikeda as genotypes that could lead to reduced health status in cattle [
4].
In the South Korea, above 95% of cattle farm are operating under non-grazed management. It is favorable for the dairy farmers as it can control outdoor conditions, reduce tick exposure, and enhance the milk yield volume and composition. In the South Korea, the most studied bovine TBD was BATOG, as previous reports revealed that it has the highest prevalence compared to anaplasmosis, rickettsiosis, ehrlichiosis, paralleled to theileriosis which has 69.4% to 96.0% prevalence in three different regions of South Korea [
5], while babesiosis was not detected based on a recent study [
6]. Recent reports on hematological changes in BATOG in South Korea, in relation to the genotypes [
4], season [
1], management and breed [
5], suggests that outbreaks in the country are mainly asymptomatic or subclinical cases only [
5]. With regards to this, we report the first clinical case of BATOG in a dairy cow raised under non-grazed farm in the upper part of South Korea.
In May 2019, the referring veterinarian had reported a Holstein-Friesian cow, exhibiting lameness, fever, inappetence, and mucosal pallor (ID: AW133). These manifestations were reported to have occurred for the past 2 years, with no proper diagnosis. The dairy farm operates under an indoor/non-grazed management, located on a hillside with bush overgrowth, on the outskirt of city of Pyeongtaek, Gyeonggi Province. The cow was 45 days in milk (high milk yield period) on its fifth parity, and was 7 years old during the clinical manifestation. Whole blood and serum was collected using ethylenediaminetetraacetic acid and serum tubes through the tail vein, and were analyzed for hematology and serum biochemistry using IDEXX ProcyteDx and IDEXX Catalyst One (IDEXX Laboratories Inc., USA), respectively. Giemsa-Wright stained blood smear was also prepared for microscopy. DNA was extracted using QIAamp DNA kit (Qiagen, Germany). Polymerase chain reaction (PCR) assays were used to detect piroplasmosis (
Babesia and
Theileria), and
Anaplasma marginale using specific region of piroplasm 18S rRNA gene [
7], and
Anaplasma major surface protein gene [
8], respectively.
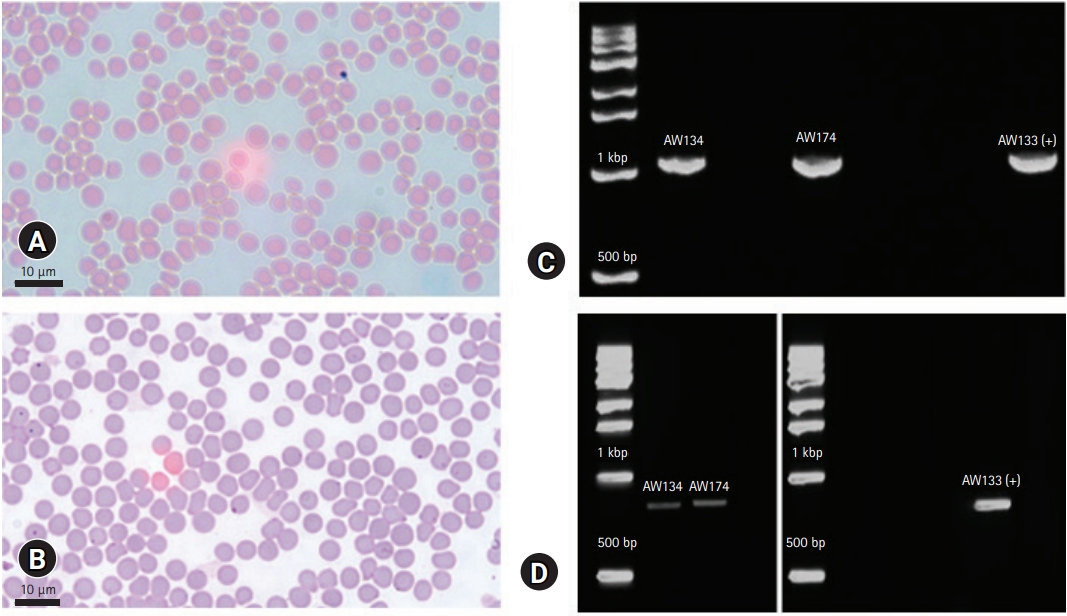
Initial results showed below normal values of RBC and certain RBC parameters, high total bilirubin (TBil) (
Table 1). Microscopy showed no hemoparasites, but anisocytosis was observed (
Fig. 1). The case was confirmed piroplasm-positive based on PCR (
Fig. 1C). Then, surveillance was conducted to all the lactating cows from the same farm after the release of the initial results of AW133. A total of 40 whole blood samples were collected from asymptomatic lactating cattle and were tested as above. Among the animals, 2 (ID: AW134, AW174) showed low RBC count (
Table 1). These were also positive for piroplasmosis, and negative for anaplasmosis. Stained blood smear also showed anisocytosis, with no hemoparasite observed. To identify whether
Theileria or
Babesia is involved (including AW133), genus-specific regions of 18S rRNA were PCR amplified, revealing these animals have theileriosis. To further identify the
Theileria genotype, major piroplasm surface protein gene (
mpsp) was amplified (
Fig. 1D) following published protocol [
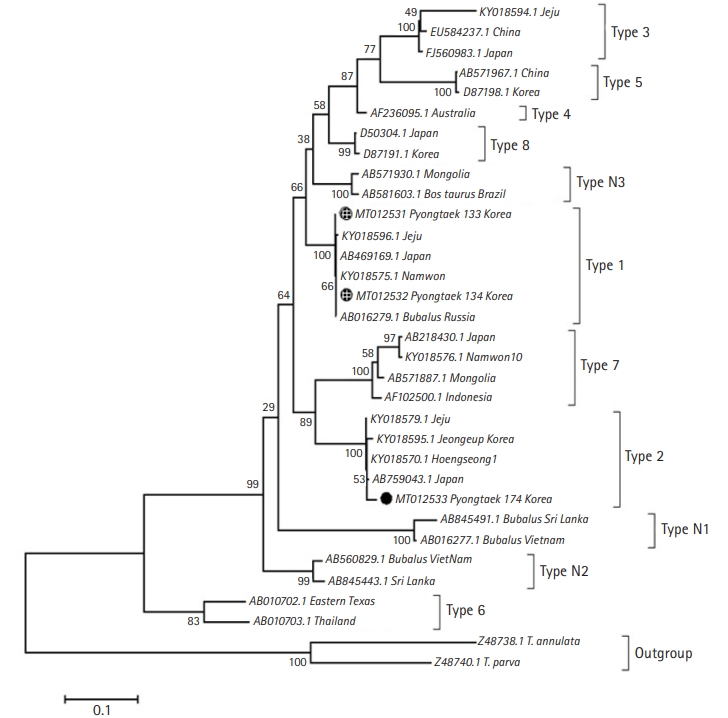
9], then sequenced for genotyping. Phylogenetic analysis identified the clinical and one asymptomatic case as Type 1 (Chitose), while the other was identified as Type 2 (Ikeda) (
Fig. 2). The infected cows were immediately treated with 1 mL 5% Buparvaquone (Butalex, MSD Animal Health, Egypt) per 20 kg body weight (intramuscular injection), twice (48-hour interval). Two weeks later, the clinical case recovered, and RBC returned to normal levels.
This study reported a rare clinical case of BATOG in non-grazed dairy cow in the upper part of South Korea, where no previous case was reported. The cause of the clinical presentations was unknown, therefore, a potential hemoparasitism-causing anemia and hyperbilirubinemia on AW133 was hypothesized. Upon piroplasmosis confirmation, a follow-up surveillance on all lactating cows from the same herd was conducted. Lastly, the T. orientalis genotypes were identified.
Low RBC was detected on the clinical, and on 2 asymptomatic cases, indicating anemia or hemorrhage. Hemolytic anemia is a regenerative bone marrow response associated with hemoparasitism and was considered as an initial diagnosis for theileriosis [
3]. This is because piroplasmosis causes destruction of RBC [
2], linking AW133’s high TBil level. After hemolysis, hemoglobin escapes into the bloodstream, binds to haptoglobulin/hemopexin, then taken up by macrophages/hepatocytes which transform it to unconjugated bilirubin. The more unconjugated bilirubin is produced than the liver can handle, the higher bilirubin values can be measured. Lawrence et al. [
10] have observed the same pattern of increased bilirubin in anemic
T. orientalis-infected cattle in New Zealand.
The microscopy of stained-blood which showed no parasitemia can either be due to the early phase of infection or a recovered acute infection that sustained subclinical infection, which are both microscopically undetectable [
11]. Anisocytosis or unequal RBC size is a commonly observed morphological change of RBC in animals with regenerative anemia [
10]. Oakes et al. [
3] also observed this in cows infected with
T. orientalis, alongside the increase in TBil.
Phylogenetic analysis of
mpsp of the samples revealed that Type 1 and 2 were present within the farm. Previous studies suggested that Type 2 is the key causal genotype for clinical
T. orientalis infection. But in a recent study, cattle with Type 1 infection showed a higher number of cattle with lower RBC, compared to those infected with other genotypes [
4]. However, though RBC and RBC-related indices were lower to those that are non-infected, the values were still within normal range [
1,
5]. The presence of multiple genotypes in a herd is not a rare occurrence. Previous studies reported that vector ticks in a specified area could harbor three pathogenic genotypes of
T. orientalis [
12], and cases of mixed infection do not always lead to a clinical disease [
13].
In South Korea, subclinical BATOG was often reported in native beef cattle, Hanwoo [
4,
5], and Holstein breed were reported to be more susceptible [
14], but limited studies are available [
1,
15]. The low percentage prevalence of theileriosis observed in this report could be due to the non-grazing management, because grazing cattle are more exposed to ticks in pastures. Kim et al. [
5] in 2017 previously reported that theileriosis in grazed Hanwoo has higher prevalence (56.6%) compared to non-grazed (17.6%). In this study, tick was not observed on the infected animals. Thus, it is not definite how these animals obtained BATOG, but it was presumed that vector ticks could have originated from the hillside bushes around the farm. Also, the warm weather during the transitioning of spring to summer in May could have also attributed in increased tick distribution, as during this month was reported to be the start of an increasing distribution of
Haemaphysalis spp. in upper South Korea [
1,
16]. Furthermore, the effect of climate change on the re-emergence of TBDs, especially in temperate countries like South Korea, could have also aided on the disease incidence. However, due to the absence of ticks in the herd, the possibility of transmission via other mechanisms such as husbandry practices, transplacental and colostral transfer, are also likely, as suggested by Lakew et al. [
17] who investigated on endemic multiple genotype infection of
T. orientalis, despite limited presence of ticks. Additionally, the clinical manifestations of BATOG to AW133 could have interplayed with the weakened immunity due to age, lactation period, and parity. These factors could contribute to stress leading to immunosuppression, worsening the symptoms. Moreover, warm weather can also increase the pathogenic effect of BATOG [
18]. The 2 asymptomatic cases could have been a long-term carrier of
T. orientalis and already developed immunity.
This is the first report of a clinical case of BATOG in non-grazed dairy cow in South Korea, specifically at the upper part of the country. Clinical cases of BATOG were rarely reported, especially in non-grazed dairy cattle, as reports in South Korea were subclinical manifestations only. Asymptomatic BATOG cases left untreated could serve as parasite reservoir [
6], which could lead to significant production losses, especially in non-grazed animals confined in limited space. With the effects of climate change, indoor-raised animals are now becoming at higher risk of TBDs which were previously under control in non-grazing system. Farm management adaptations to TBD emergence, fluctuating climate, and seasonal tick control, accompanied with regular animal health monitoring, are efficient means for early prevention of the severity and spread of this disease.









 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



