Prophylactic effect of topical betaxolol and dorzolamide on the fellow eye in unilateral canine primary angle closure glaucoma: 60 cases (2016.1-2021.5)
Article information
Abstract
This study was aimed to evaluate the prophylactic anti-glaucoma effect of topical 5% betaxolol (BTX) and 2% dorzolamide (DRZ) on the second eye in dogs with unilateral primary angle closure glaucoma (PACG). Medical records of 60 dogs with unilateral PACG who received prophylactic anti-glaucoma eyedrops in the second eye, from 2016 to 2021, were reviewed. The prophylactic effects of BTX were maintained on 28/60 (46.7%) eyes until last visit and BTX failure was observed on median 510 (range, 53-1,927) days in 32/60 (53.3%) eyes. After DRZ instillation in BTX failure eyes, the prophylactic effects were extended at median 610 (range, 157-2,270) days in 21/32 (65.6%) eyes. DRZ failure eyes (17/21, 81.0%) eyes required chemical ablation or surgical intervention due to uncontrolled intraocular pressure. The duration of prophylactic effects was decreased with aging (R2 = 0.334, p = 0.006). The predominant breeds were Shih-Tzu (41.9%) and American Cocker Spaniel (30.6%) with no significant differences in survival curves (p = 0.210). The average prophylactic effects of BTX persisted more than 1.5 year and could be selected the first prophylactic eye drop in unilateral PACG. Also, early surgical intervention should be considered in prophylactic medications failure cases.
Introduction
Glaucoma is a progressive and neuro-degenerative ocular disease [1,2], influenced by elevations of intraocular pressure (IOP) which accelerates retina and optic nerve damages leading to irreversible blindness [3,4]. Glaucoma can be classified as primary or secondary [5]. Primary glaucoma is further divided into primary angle closure glaucoma (PACG) and primary open-angle glaucoma (POAG) [5], which can be distinguished using ultrasound biomicroscopy (UBM), gonioscopy, or histopathology [6]. The POAG is characterized by an originally normal-appearing iridocorneal angle (ICA) on gonioscopy and a gradual bilateral elevation of IOP in 1 to 3 year-old dogs [3,6]. The PACG is 8 times more common than POAG and on gonioscopy may appear as an ICA, which appears narrowed, partially closed, or comprised of pectinate ligaments dysplasia (PLD) initially, and is virtually completely closed when IOP is severely elevated [6]. The PACG tends to represent middle-aged to older dogs of predisposed breeds [7]. A previous study showed that canine PACG usually occurs in the fellow eye with initially normal IOP at a median of 8 months after the disease becomes apparent in the first eye [7]. Considering the high risk of glaucoma and the low success rate in preserving vision in the fellow eye, prophylactic therapy is recommended for them [1,8].
In few studies, veterinary ophthalmologists had initiated prophylactic medication in the normotensive fellow eye following diagnosis of glaucoma in the first eye [1,8]. Some retrospective studies using different treatment protocols tried to evaluate the efficacy of prophylactic therapy in the normotensive fellow eye [1,8]. However, a recent survey showed that not only the studied eye drops but also various other medications have been used by veterinary ophthalmologists [1,8]. In previous studies, topical 0.5% timolol maleate has shown ocular hypotensive effects [9-11]. In addition, few studies have shown the IOP lowering effect of beta-blocker in animals [12,13]; among them, one study reported that 0.5% betaxolol (BTX) lowered IOP in unaffected dogs [13]. However, the hypotensive effect of BTX is weak, and it is not recommended alone to lower IOP [5]. Although a recent study showed the ability of 0.5% BTX to delay glaucoma in the fellow eye in canine unilateral PACG, its detailed prophylactic effect in dos are unknown [7].
Dorzolamide (DRZ) hydrochloride is widely used to lower IOP [14]. In the ciliary process, inhibition of carbonic anhydrase isoenzyme II results in decreased secretion of aqueous humor by up to 50%, with subsequent reduction in IOP [5,15]. Therefore DRZ is not only used in the management of acute glaucoma crises and long-term control of IOP in patients, but may also be used in glaucoma prophylaxis [5].
This study was conducted to identify the effects of BTX and DRZ on the fellow eyes of canine PACG patient and to determine the prognosis of prophylactic glaucoma therapy. In addition, this study aimed to determine whether the period of glaucoma prevention could be extended when changing to the second topical prophylactic anti-glaucoma medication, after failure of the first.
Materials and Methods
This retrospective research was based on data from canine PACG presented between January 2016 and June 2021. The clinical data of patients that developed PACG in the first eye and received prophylactic treatment in the fellow eye, were reviewed. Veterinary ophthalmologists confirmed PACG after reviewing ophthalmic examination and clinical history.
All dogs underwent a complete ophthalmic examination, including rebound tonometry (TonoVet; Icare Finland Oy, Finland), indirect ophthalmoscopy (Vantage Indirect Ophthalmoscope; Keeler, UK), and slit-lamp biomicroscopy (SL-D7; Topcon Corp., Japan). The diagnosis of PACG was based on the clinical history and abnormal ICA in the normotensive fellow eye that was narrow or closed and/or had a PLD. When PACG was confirmed in one eye, the fellow eye was examined by gonioscopy (Volk Optical, USA) and UBM (50 MHz transducer, MD-320W; MEDA, China). Patients diagnosed with open-angle or secondary glaucoma were excluded in this study. Also, patients with a history of intraocular surgery, moderate to severe uveitis, mature/hypermature cataract, and other intraocular diseases that could affect the outflow of aqueous humor, were excluded [5,16].
Clinical data were confirmed for patients with a follow-up period of more than 6 months. For the prophylactic treatment, 0.5% BTX (Betoptic; Alcon Laboratories Inc., USA) was applied twice a day in all patients. After BTX failure to control IOP, BTX was changed to 2% DRZ (Trusopt Santen Pharmaceutical, Japan) twice a day to extend the prophylactic medication period.
In this study, all IOP were evaluated at each visit, and the criteria for patients were based on maintaining IOP, vision and clinical signs of glaucoma until the last visit by regular recheck. BTX treatment failure was defined as IOP more than 20 mmHg and changing topical prophylactic medicine to maintain normotensive IOP (BTX to DRZ) without visible retinal/optic disc damage in fundoscopy. DRZ treatment failure was defined as uncontrolled IOP (> 20 mmHg) with or without abnormalities in the retina and optic disc, and other ophthalmic abnormalities related to glaucoma (corneal edema, mydriasis, episcleral injection, and buphthalmos).
Clinical data collected from each patient included sex, breed, age, dates of onset of first eye glaucoma, dates of onset of treatment failure in the fellow eye (BTX or DRZ treatment failure), glaucoma prognosis following that, and side effects of these medications.
Statistical analyses were performed using the Statistical Package for Social Sciences ver. 26.0 for Windows (IBM Corp., USA). Kaplan-Meier method and Breslow and Mantel-Cox test were used to compare survival curves between breeds with a sufficient number of patients. In the multiple linear regression analysis, the partial regression coefficient was used to show how much change could be expected from the dependent variable. The R2 values represent the explanatory variables.
Results
A total of 60 eyes of 60 dogs were studied, consisting of 38/60 (63.3%) females (10 intact/28 neutered) and 22/60 (36.7%) males (2 intact/20 neutered). The median age of the dogs was 10 years (range, 5-16 years). The right eyes (n = 28, 46.7%) and left eyes (n = 32, 53.3%) were almost equally affected.
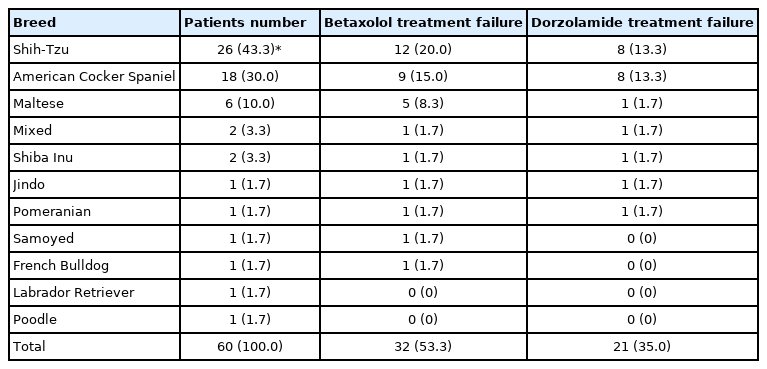
Eleven breeds of dogs were included in this study (Table 1). Shih-Tzu (n = 26/60, 43.3%) were the most common breed, followed by American Cocker Spaniel (n = 18/60, 30.0%), Maltese (n = 6/60, 10.0%), and other breeds (n = 10/60, 16.7%) (Table 1).
Twenty-eight/sixty fellow eyes (46.7%) had normal IOP and were checked regularly during BTX instillation. However, 32/60 fellow eyes (53.3%) showed high IOP median of 510 days from the first glaucoma onset (range, 53-1,927). Because the eyes showed high IOP, BTX was changed to DRZ to prolong the prophylactic period. On median 610 days from the first glaucoma, 21/32 fellow eyes (65.6%) developed high IOP during DRZ instillation indicating (range, 157-2,270), and the rest 11/32 fellow eyes (34.4%) retained normal IOP until last visit (Fig. 1).

Graphical flow chart showing patient distribution by prophylactic medication changes. BTX, betaxolol; IOP, intraocular pressure; DRZ, dorzolamide.
All DRZ failure 21 eyes were diagnosed with glaucoma, present in with high IOP/impaired vision and were administered additional anti-glaucoma drugs (prostaglandin analog eye drops; 0.005% latanoprost or 0.03% bimatoprost). Of these 21 fellow eyes, 16 eyes in which IOP could not be controlled with additional topical anti-glaucoma medications, lost their vision, underwent salvage procedures with cidofovir chemical ablation. In 1 eye, although vision was still confirmed, IOP was not controlled with topical anti-glaucoma medications and therefore, gonio implantation was performed. In other 4 eyes, though still suffered from impaired vision, IOP was controlled with topical anti-glaucoma medications (Table 2).
In case of BTX treatment failure, Shih-Tzu comprised the highest number of dogs i.e., 12, followed by American Cocker Spaniel with 9 dogs. Eight Shih-Tzu and American Cocker Spaniel dogs progressed to DRZ treatment failure (Table 1). Shih-Tzu developed glaucoma in the fellow eye a median of 692 days after onset of PACG in the first eye (mean, 928.6 ± 686.7; range, 53 to 2,342 days; 95% confidence interval [CI] for the median time to the onset of glaucoma, 1,287.7 to 2,011.0 days). American Cocker Spaniels developed glaucoma in the second eye at a median of 512 days after the onset of PACG in the first eye (mean, 715.6 ± 596.3; range, 63 to 2,270 days; 95% CI, 722.0 to 1,595.2 days). Although the period of development from the first eye to the second eye in Shih-Tzu was longer than that in American Cocker Spaniel, there were no significant differences between the survival curves for the Shih-Tzu and American Cocker Spaniel (Mantel-Cox, p = 0.17) (Figs. 2, 3).

Kaplan-Meier survival curve comparing by breeds treating the second eye with 0.5% betaxolol twice a day, in 44 dogs (Shih-Tzu [ST], 26 dogs; American Cocker Spaniel [ACS], 18 dogs) with primary angle closure glaucoma. There is no significant difference between 2 breeds (p = 0.94). +: Last visit of normal intraocular pressure/vision fellow eye.
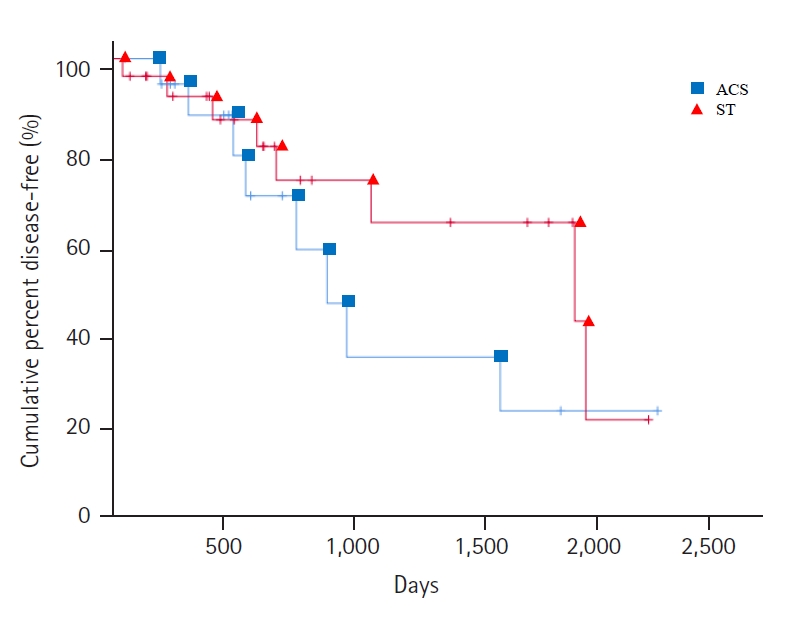
Kaplan-Meier survival curve comparing by breeds treating the second eye with 2% dorzolamide twice a day after 0.5% betaxolol treatment failure, in 44 dogs (Shih-Tzu [ST], 26 dogs; American Cocker Spaniel [ACS], 18 dogs) with primary angle closure glaucoma. There is no statistically significant difference between 2 breeds (p = 0.17). +: Last visit of normal intraocular pressure/vision fellow eye.
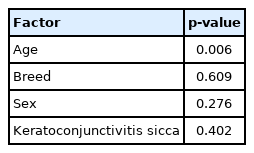
In the multiple linear regression analysis, clinical data from 21 dogs that progressed to glaucoma were evaluated. This analysis was conducted to determine which factors were highly correlated with the occurrence of glaucoma in patients undergoing prophylactic treatment, including sex, breeds, age, duration of prophylactic treatment, and presence of other ophthalmic diseases. In the multiple linear regression analysis, when glaucoma prophylactic medication was initiated, it was confirmed that the prophylactic medication treatment date was related to age (p = 0.006, R2 = 0.334). Each time the age at which prophylactic medication was started increased by a year, the treatment period decreased by 112 days [Prophylactic medication treatment date (days) = 1,882-112 × Age (years)]. Other factors (breeds, sex, keratoconjunctivitis sicca [KCS]) did not affect the treatment period in eyes with glaucoma (Table 3).
Discussion
Glaucoma is a complicated neurodegenerative disease that represents the eventual common pathway of many ophthalmic diseases [3]. Although a pathologically high IOP is considered a priority risk factor, there are many other factors are also important in the disease pathogenesis [3]. Retrograde axonal transport is decreased by changes in the scleral lamina cribrosa, such as subsequent retinal ganglion cell death and neurotrophin deprivation [17]. Hypoxic-ischemic neural damage is caused by high IOP exceeding the ocular perfusion pressure, which leads to decrease blood flow to the optic nerve and retina [18]. This condition also causes additional damage to the retina and optic nerve, even if it prevents further reperfusion damage by lowering IOP by amino acid excitotoxicity, abnormal gene expression following the accumulation and formation of reactive oxygen species [19–21]. These features of glaucoma support the need for prophylactic anti-glaucoma medication. Previous study has documented that, without prophylactic anti-glaucoma treatment, a high proportion of PACG patients develop glaucoma in the contralateral eye; therefore, attempts have been tried to delay the onset of glaucoma in the normotensive fellow eye [8]. Many studies suggested that prophylactic topical applications could delay the onset of glaucoma in the normotensive fellow eye [7,8].
BTX, a selective beta-blocker, reduces IOP in normotensive canine eyes [13]. However, beta-blocker eye drops as sole topical drugs are weak and have certain limitations for glaucoma therapy in dogs [5]. However BTX may have a role in glaucoma prophylaxis in dogs [5,7]. DRZ, a carbonic anhydrase inhibitor has been shown to reduce the IOP in normal and glaucomatous eyes [22]. A previous study on several specific prophylactic drugs studied the effect of 2% DRZ hydrochloride, 1% brinzolamide, or a combination of 2% DRZ hydrochloride with 0.5% timolol in delaying the elevation of the IOP in the second eyes of dogs with PACG. This study also showed that the duration of glaucoma in the second eye occurred 15.7 months after instilling DRZ in the first eye [23].
A previous study conducted to investigate the breed distribution according to primary glaucoma, confirmed that American Cocker Spaniel and Chow Chow were the most predisposed breeds [24]. However, in the current study that included only PACG patients, American Cocker Spaniel and Shih-Tzu were the most predisposed breed. This might be due to a change in the preferred breed with time. Similar to previous studies, American Cocker Spaniel was the second most popular breed in the present study (31.7%) [7,8,23–25]. However, Shih-Tzu was the most represented breed in this study. As in other studies, PACG was diagnosed more frequently in females (63.3%) than in males in this study [23,26]. In this study, most patients were middle-aged to older dogs (median 10 years) as in previous studies [3,5].
In this study, there was no control group as all the patients received prophylactic treatment for glaucoma. The median time of onset of glaucoma in the fellow eye of patients that received prophylactic treatment in this study was longer (20.3 months) than in the control group in the previous study (median, 8.0 months) [7]. When compared with the prophylactic treatment group in the same study, the results of this study were shorter (median, 30.7 months) [7]. The difference in these results was thought to be due to the difference in breed distribution [27]. After BTX treatment failure, although the duration of the prophylactic treatment period was extended with DRZ, glaucoma occurred in most BTX failure patients (n = 21, 65.6%). In addition, in eyes with DRZ failure, IOP was not controlled further in most patients (17/21), even additional ant-glaucoma drugs. Therefore, after confirming an elevated IOP during DRZ instillation, if prostaglandin is prescribed, its effect on IOP levels should be monitored in the second eye and a surgical plan should be considered accordingly [28]. Of the 60 dogs, 28 (46.7%) dogs maintained stable IOP only by instillation of BTX. Among 32 dogs with BTX treatment failure, when the prophylactic medication was changed to DRZ, n-IOP was maintained in 11/32 dogs (34.3%) until the last visit. In 4 out of 21 dogs (19.0%) with DRZ treatment failure, IOP was controlled with additional anti-glaucoma medications. In the previous study, 50% of PACG dogs that received prophylactic medications maintained a normotensive IOP [23]. But in this study, 65% of dogs maintained prophylactic medication. The increase in the success rate in this study was higher than the previous study [23], which was thought to be due to change the prophylactic medication; BTX to DRZ due to stronger anti-glaucoma effect of DRZ than BTX.
Although there was a difference in the mean until the onset of glaucoma in the fellow eye of the Shih-Tzu and American Cocker Spaniel, there was no significant difference in the survival curve comparison. This result might imply that the incidence of fellow eye glaucoma in PACG was not breed-dependent. However, there was a limitation in that comparisons were not made between various species and among more patients.
The multiple linear regression test confirmed that the prophylactic medication period decreased with increasing age. These results were thought to be influenced by age because ICA and CC progressively narrow in PACG patients [5]. Recent retrospective studies have also reported that patient age is directly proportional to incidence of post-operative glaucoma [29,30]. These results support the results of other studies suggesting that prophylactic glaucoma medications should be administered immediately in the second eye after confirming of PACG in the first eye [1,5,7,8,23,26].
There were 36 eyes diagnosed with KCS, or in which treatment equivalent to KCS was required during BTX instillation. In a previous study, a relatively high incidence of KCS was noted in dogs in the BTX treatment group during the data analysis [7]. Not only the average age of the dogs included in this study was old (10.1 ± 3.3 years) but most of the patients were of certain breeds (American Cocker Spaniel, Shih-Tzu), and these breeds were reported as KCS predisposing breeds in previous studies [31,32]. In addition, there was no control group, so the existence of other effects could not be excluded. However, no association was observed between the duration of BTX instillation and the occurrence of KCS in this study (p = 0.38).
The limitations of this retrospective study were the small sample size and the absence of a control group. In addition, because of the specific breed preference, it was difficult to compare by breeds. This precluded the ability to analyze differences between the type and frequency of anti-glaucoma medication administrate or if anti-glaucoma therapy extended the median time of medication failure. When IOP spikes were checked in the second eye, cases of uveitis in the anterior chamber were excluded, which may have affected the results [3–35]. Additional controlled studies with larger sample sizes would be needed to investigate the use of topical anti-glaucoma medications to delay or prevent the onset of canine PACG.
In conclusion, in a patient with PACG in the first eye, glaucoma developed in the second eye at a median of 610 days after instillation of BTX to DRZ. The use of BTX followed by DRZ was not enough preventive effect on the fellow eye glaucoma incidence in this study. Therefore, surgical interventions or therapeutic prostaglandin analog instillation should be necessary to maintain the vision of the second eye. In addition, the duration of prophylactic glaucoma medications in the second eye decreased with age. Therefore, immediate prophylactic medication for the second eye should be important as soon as PACG is detected in the first eye.
Notes
The authors declare no conflict of interest.
Acknowledgements
This study was supported by BK21 FOUR Future Veterinary Medicine Leading Education and Research Center and the Research Institute for Veterinary Science (RIVS), College of Veterinary Medicine, Seoul National University, Seoul 08826, Republic of Korea. In addition, this research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2021R1I1A1A01058695).