The main reasons for wildlife rescue are trauma secondary to collisions, accidents, poaching, and predator attacks [
1]. Trauma commonly causes open wounds; consequently, wound management is an important aspect of treating wild animals. Wild mammals are treated according to usual wound management procedures; however, owing to the various characteristics of wild animals (high aggression and stress susceptibility), a more rapid problem-solving procedure is required [
2,
3].
As open wounds are often accompanied by wound site infections, appropriate infection evaluation and antibiotic selection are important for prognosis. The antibiotic susceptibility test (AST) is an essential method for selecting antibiotics and detecting antibiotic resistance in bacteria isolated from wound sites. The Kirby-Bauer disk diffusion test is widely used in veterinary clinical settings because of its simplicity and rapid performance [
4]. In particular, owing to the nature of wild animals with low skin cleanliness, it is important to select and replace antibiotics through tests because bacterial infection at the wound site can change over time. In addition to the overuse of antibiotics, their inadequate selection and administration lead to antimicrobial resistance and introduction into the environment such as water and soil, resulting in their transmission to humans and domestic animals [
5,
6].
In this report, we describe the successful treatment of a wild raccoon dog with a severe open wound and secondary bacterial infection by applying active drainage and repeated ASTs. In particular, owing to the characteristics of wild raccoon dogs, modified active drainage was performed at an early stage in the presence of multidrug-resistant (MDR) bacterial infection and tissue necrosis, which were successfully treated through regular ASTs and infection management.
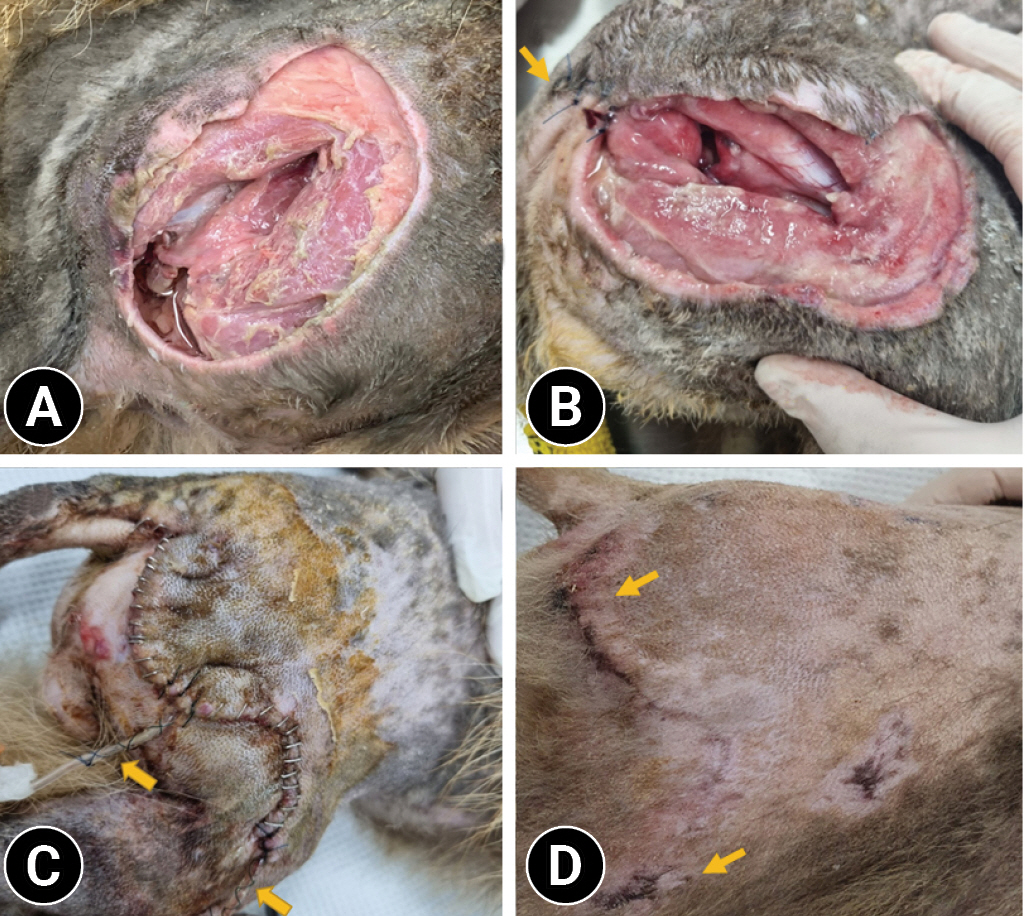
An adult male raccoon dog weighing 5 kg was rescued after being hit in a vehicular accident. On physical examination, the raccoon dog showed decreased activity but responded to external stimuli. An open wound approximately 10 cm in size was observed on the right thigh, and the exposed femoral head was fractured (
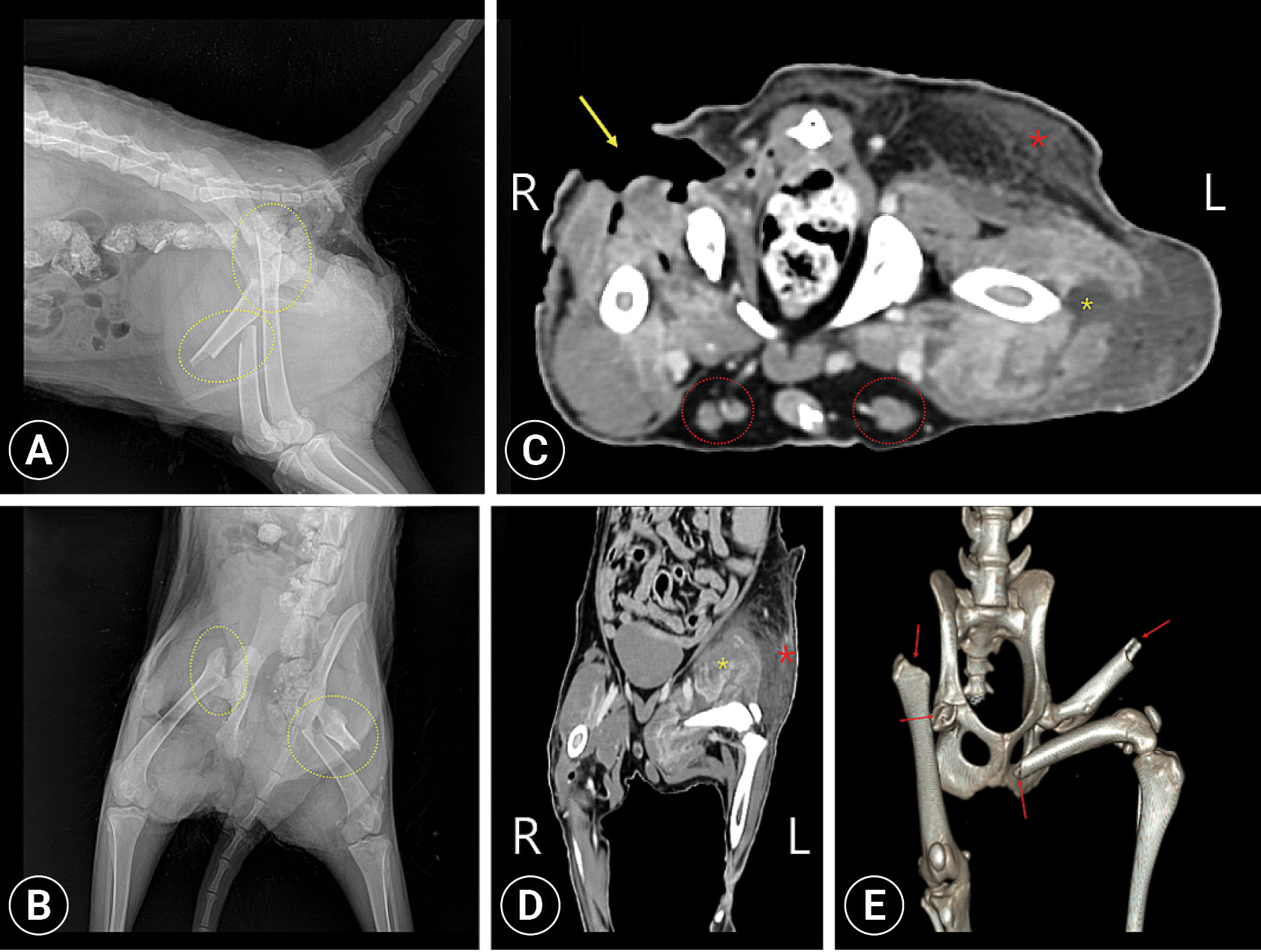
Fig. 1A). The open wounds were heavily contaminated with maggots. The typical hyperkeratosis caused by scabies was observed throughout the body. Complete blood count (CBC), serum biochemistry panel, and blood gas analysis showed mild acidosis, hypernatremia, and increased alanine aminotransferase levels. Radiography and computed tomography (CT) were conducted to evaluate fractures and other lesions (
Fig. 2). Through the imaging evaluations, complete fracture of right femoral neck and left femoral diaphysis were observed, and additional fractures of the right acetabulum and the transverse process of the 1st caudal vertebrae were observed. After contrast enhancement of CT, the thigh muscles on both sides were observed heterogeneously, indicating myositis, and the left side was more severe. Hematomas were seen in the left subcutaneous (SC) gluteal region and hind limbs. In the left hindlimb, some trajectories of various vessels, including the saphenous artery, were not observed, suggesting that later ischemic disease may be present. Bilateral superficial inguinal LN enlargement was also observed. Taken together, the raccoon dog was diagnosed with extensive open wound and multiple fractures. The raccoon dog was administered cefazolin (Cefazoline Injection 1 g; Chongkundang, Korea) at 30 mg/kg intravenous (IV) and enrofloxacin (Baytril; Elanco, USA) at 5 mg/kg IV, ivermectin (SI Ivermectin-premix; SB Shinil Co., Ltd., Korea) at 0.024 mg/kg SC, meloxicam at 0.1 mg/kg IV (Metacam injection; Labiana Life Sciences SA, Spain) and butorphanol at 0.1 mg/kg IV (Butophan Inj; Myungmoon Pharm Co., Ltd., Korea) along with fluid therapy, and was stabilized. After confirming the patientŌĆÖs appetite, it was converted to oral medicine.
A day later, the wound on the right thigh was cleaned and debrided under general anesthesia. To reduce fecal contamination, approximately 3 cm of the skin near the anus was temporarily closed using a continuous suture (
Fig. 1B) and then bandage was constructed in 3 layers (gauze-cotton material-elastic self-adherent material). Thereafter, daily flushing using normal saline drawn into a 20 mL syringe and the wound is lavaged with an 18-gauge needle and non-surgical debridement of the wound area were performed. All fractured regions in both femurs, 1st coccygeal area, and right acetabulum were stabilized with splints to prevent additional soft tissue damage.
Macrococcus spp. and
Proteus spp. were isolated from wound culture and AST performed on the day of admission, which showed that the selected antibiotics were sensitive and therefore, maintained. Blood analysis, especially CBC, were continuously conducted for patient evaluation. On the third day after rescue, the test results showed severe anemia (packed cell volume, 17.7%) and leukocytosis (29,360 cells/╬╝L). Hypernatremia was also seen. However, 1 week after admission, the left hind leg underwent amputation due to sensory loss and necrosis at the bottom of the fracture.
After 3 weeks, the activity and aggression of the raccoon dog increased significantly, and it became difficult to keep the wound open on the right thigh. Although the infection and tissue necrosis remained, modified active drainage was performed using two 18-G scalp needles with a plain tube, and the wound on the right thigh was closed with sutures in the order of muscle, SC tissue, and skin (
Fig. 1C). The fractured femoral head was removed before wound closure. The amount of exudate in the tubes was measured and aerobic and anaerobic bacterial cultures were performed daily to monitor the level of bacterial infection. Blood tests showed only severe leukocytosis (31,230 cells/╬╝L) and mild anemia (28.8%)
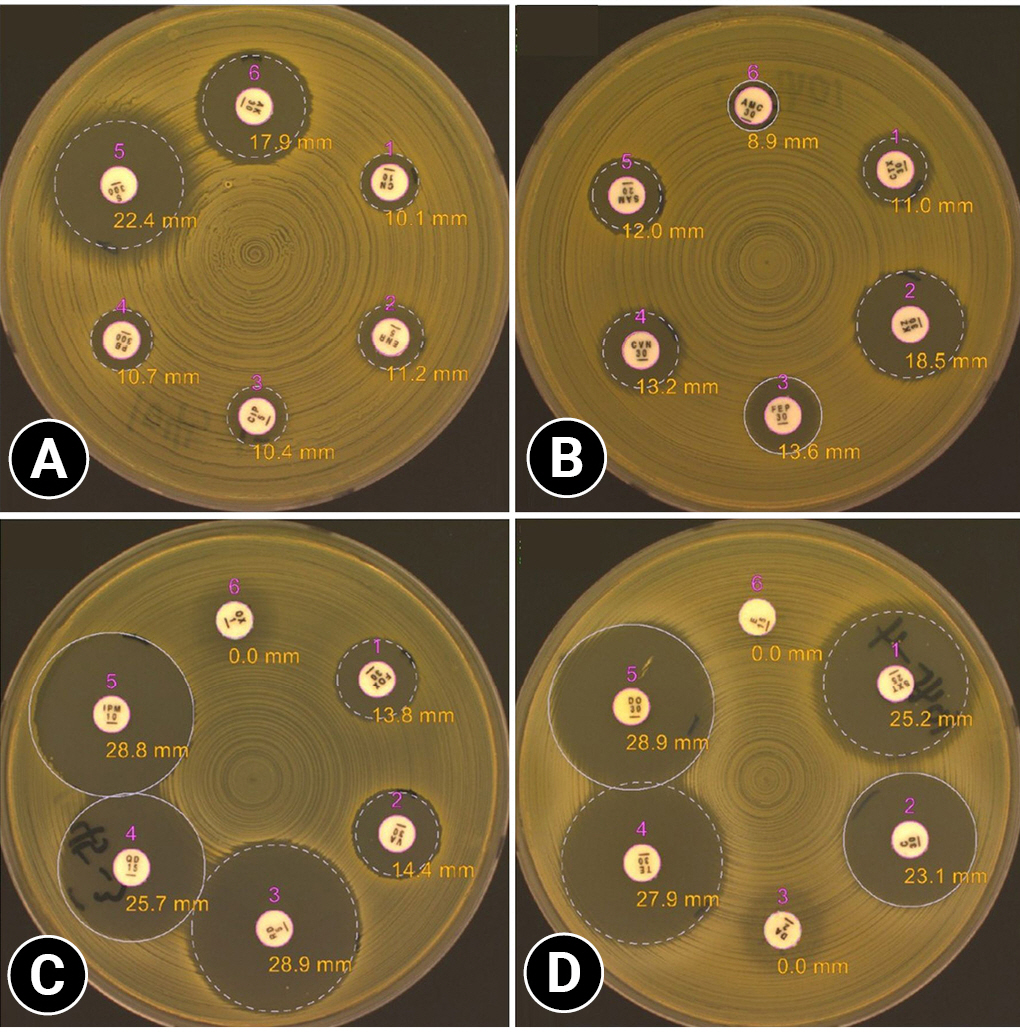
Simultaneously, AST was performed weekly for monitoring the wound for instead of and, site condition and selecting an appropriate antibiotic, after the first presentation. Swab samples or exudates in the tubes (after installing active drainage) were cultivated aerobically and anaerobically on trypticase soy agar containing 5% sheep blood. The Kirby-Bauer disk diffusion test and E-test (for glycopeptide-resistant bacteria) were performed according to Clinical and Laboratory Standards Institute guidelines (M100-Ed32). To effectively control the infection, antibiotics were changed to the most sensitive ones in the test results (
Supplementary Table 1). Initially, amoxicillin/clavulanic acid and metronidazole were administered, which were then changed to trimethoprim/sulfamethoxazole, which was finally changed to doxycycline; after antibiotic change, CBCs showed a marked decrease in the white blood cell count (from 31,230 cells/╬╝L to 18,380 cells/╬╝L). The amount of exudate (from 22.8 mL to 2.2 mL) and the number of colonies (from too numerous to count to 2 colonies) were also noticeably decreased. Among the isolated
Staphylococcus spp., the first isolates on day 28 were sensitive to most antibiotics, but the isolates 5 days later (day 33) were MDR bacteria. Polymerase chain reaction targeting the chaperonin 60 (
cpn60) gene sequence revealed that the first isolated
Staphylococcus spp. was
Staphylococcus pseudintermedius (SP), but the latter isolates were identified as
Staphylococcus aureus (SA) [
7]. Finally, 5 weeks after the start of treatment, the patient's lesion was cured and the patient was released after a month of rehabilitation (
Fig. 1D).
Supplementary Table 1 summarizes the genera of the isolated bacteria and the selected antibiotics according to the AST results and
Fig. 3 was example of an AST results.
Open wound management in wildlife is common but is difficult because of their characteristics and circumstances, such as severe contamination or necrosis of lesions, delay in treatment, aggression, and a hospitalized space that is difficult to keep clean. As time is inevitably delayed when injured wildlife are found and transported to the wildlife rescue center, the probability of contamination or infection in the lesion also increases. A drain is useful for removing fluid or air from a wound. However, the inappropriate use of drains can cause side effects such as increased infection or delayed healing, especially when contamination is present and necrosis is progressing [
8]. The patientŌĆÖs wound was cleaned and dressed daily, but there was difficulty in managing it due to continuous contamination as the lesion was close to the anus, and the space where the patient was hospitalized was narrow and difficult to keep clean. Aggression is also a difficult factor in wound management. Therefore, we decided to place an active drain and measured the volume of exudate and the number of colonies daily to monitor the infection. Initially, the volume of the exudate was approximately 10 to 25 mL, and the number of colonies was too large to count. However, after a week, the amount of exudate decreased noticeably (under 5 mL), and the number of colonies also dropped sharply, reaching less than 10. The drain was removed 15 days after placement, and the wound healed without any side effects.
Systemic antibiotics are essential in cases with dirty wounds or suspected infections. Optimal selection of antibiotics is essential for controlling infection and healing [
8]. Wildlife is considered to contribute not only to infectious diseases but also to the spread of antibiotic resistance genes [
9,
10]. This not only threatens public health but also affects the treatment of injured wildlife [
11]. SP and SA are opportunistic infectious bacteria [
12]. In this case, SP and SA were isolated after the start of treatment. The SP isolate was susceptible to many types of antibiotics, whereas the SA isolate was MDR. In addition,
Macrococcus spp. isolated on the first day of treatment were susceptible to many antibiotics when isolated 3 weeks after treatment. It was difficult to discuss nosocomial MDR in this study. Additional research on nosocomial MDR, including the present case, is currently being conducted in our laboratory. Unlike companion animals, there are no guidelines for the selection and treatment of appropriate antibiotics in wildlife [
13]. Additionally, wildlife may have antibiotic resistance genes which can be overlooked, making treatment more difficult [
14]. In the case of this patient, considering that the important thing in the initial treatment of open wound is to control the infection, it can be seen that the infection was effectively controlled through appropriate laboratory tests.
In summary, this case showed that extensive and open wounds were effectively treated with regular dressing, active drainage, and appropriate antibiotics. Choosing the best method to manage open wounds in wildlife is challenging. Future research should continue to determine the most effective method of wound healing in wildlife.











 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement
Supplement Print
Print



