Protective effect of platelet-rich plasma against cold ischemia-induced apoptosis of canine adipose-derived mesenchymal stem cells
Article information
Abstract
This study was performed to assess the antiapoptotic effect of canine platelet-rich plasma (PRP) treated on the canine adipose-derived mesenchymal stem cells (cMSCs) under cold ischemic conditions. The effect of preventing apoptosis of cMSCs was evaluated in the apoptotic condition induced by cold ischemic injury in vitro. To determine the progression of apoptosis, the changes in cell nucleus were observed using 4',6-diamidino-2-phenylindole (DAPI) fluorescence staining. In addition, we examined the mitochondrial membrane potential (MMP) and caspase-3 activity. When the cold hypoxic injury was applied to cMSCs, the apoptotic change was observed by DAPI staining, mitochondrial staining for MMP, and caspase-3 assay. PRP significantly decreased the number of apoptotic cells. Nuclear shrinkage and fragmentation of apoptotic cells in control groups were observed by DAPI staining. The MMP was recovered by the treatment of PRP. In addition, when the luminescence intensity was measured for caspase-3 activity, the value was significantly higher in the PRP treated groups than the control groups. The results of this study showed that the PRP may have a beneficial effect on apoptosis induced by cold ischemic injury.
Introduction
Stress is known as the collapse of homeostasis, which is managed by the complex formations of physiological and behavioural adaptive responses of organisms [1]. Cold stress can disrupt the balance in the oxidant/antioxidant system and cause oxidative damage to several tissues by altering the enzymatic and non-enzymatic antioxidant status, protein oxidation and lipid peroxidation [2]. These reactions result in the elevation of oxidative metabolism [3].
The hypoxic response is a systemic process that regulates multiple cellular activities to maintain homeostasis under hypoxic conditions [4,5]. Cells undergo numerous changes in gene transcription, enzyme activities, and mitochondrial function in response to hypoxia [6]. The hypoxic response protects against stresses, but promotes disease progression in some pathological conditions [4]. On exposure to severe hypoxia beyond cellular adaptive capability, cells are irreversibly injured and die in necrosis or apoptosis [6].
Generation of oxidative stress in response to various external stimuli has been implicated in the activation of transition factors and to the triggering of apoptosis [7]. Especially the stimulation of hypoxia and ischemia may bring about the excess generation of reactive oxygen species (ROS) [8]. Cold stress also provokes ROS production [9]. ROS are produced in all mammalian cells, partly as a result of normal cellular metabolism and partly due to activation of oxidant-producing enzymes in response to exogenous stimuli [10]. Overproduction or accumulation of ROS decreases mitochondrial membrane potential (MMPs) and leads to the swelling and disruption of mitochondria [11]. Because mitochondrial ROS production under hypoxic condition increases [12], mitochondria play a major role in apoptosis triggered by such stimuli [13,14].
Apoptosis has since been recognized and accepted as a distinctive and important mode of “programmed” cell death, which involves the genetically determined elimination of cells [15]. Apoptosis also occurs as a defence mechanism such as in immune reactions or when cells are damaged by disease or noxious agents [16]. In the apoptotic process, initial stress-induced damage does not kill cells directly, rather it triggers an apoptotic signalling program that leads to cell death [14]. ROS appear to be important regulatory signals in apoptosis which are most generated by mitochondria [14,17]. They integrate and circulate death signals initiating inside the cells in regulating cell death pathways [18,19].
The cellular apoptosis occurs via mitochondria-dependent, the intrinsic or death receptor pathway and mitochondria-independent, the extrinsic pathway. Both pathways end at the point of the execution phase, considered the final pathway of apoptosis. Caspase-3 is considered to be the most important of the executioner caspases and is activated by any of the initiator caspases (caspase-8, caspase-9, or caspase-10) [15]. ROS may act as a factor initiating cellular apoptosis through a mechanism of mitochondrial pathways, which occurs with a large release of cytochrome c, activated caspase-9 and caspase-3 [18].
During the early process of apoptosis, cell shrinkage and pyknosis are visible by light microscopy [20]. With cell shrinkage, the cells are smaller in size, the cytoplasm is dense, and the organelles are mor tightly packed. Pyknosis is the result of chromatin condensation and this is the most characteristic feature of apoptosis. Apoptotic bodies consist of cytoplasm with tightly packed organelles with or without a nuclear fragment. In an intrinsic pathway, all of the stimuli cause changes in the inner mitochondrial membrane that results in loss of the mitochondrial transmembrane potential and release pro-apoptotic proteins into cytosol [15]. The reduction in mitochondrial membrane potential MMP is considered to be the earliest event during the process of cell apoptosis, before characteristics of nuclear apoptosis appear (concentration of chromatin and break of DNA). Once MMP has collapsed, cell apoptosis is irreversible [21].
Platelet-rich plasma (PRP) is a high concentration of autologous platelet derivative from whole blood, defined as concentrated plasma that has platelets above 1.0 × 106 cells/µL [22,23], and it has approximately 5 times more platelets than the normal values [24]. Close association apply between apoptosis and inflammation. For example, inflammatory mediators increase in line with apoptosis. Studies have demonstrated that PRP administration leads to an increase in the proliferation and the secretion of anti-inflammatory cytokines and growth factors [25,26], such as platelet derived growth factors, transforming growth factors β1, fibroblast growth factors, interleukin-1 receptor antagonist, vascular endothelial growth factor, and fibrinogen released by platelets [27]. These factors are thought to accelerate natural healing process and improve cartilage repair in human patients with osteoarthritis [26,28,29]. PRP also has shown beneficial effect as supporting treatment of joint problem [30], bone reconstruction [31], tendinopathy [32], articular cartilage repair [33], wound healing [34], refractory skin ulcer [35], vasculopathy [36], spinal cord injuries [37], and periodontal therapy [38], as well as decreasing inflammatory reaction and apoptosis [28].
Mesenchymal stem cells (MSCs) are the major cells isolated from bone marrow or fat which can differentiate into various kinds of cells such as adipocytes, osteoblasts, and chondrocytes in vitro and in vivo [39]. MSCs have revealed to repair tissues and suppress immune-mediated responses via paracrine secretions. However, previous studies revealed that the therapeutic methods in practice are ignoring the fact that MSCs are sensitive to concurrent O2 and serum deprivation to which they are exposed when transplanted in vivo in the hypoxic condition, which is considered as major problem in clinical application [21].
Taken together, we hypothesized that PRP would have a beneficial effect on apoptotic changes of canine adipose-derived MSCs (cMSCs) through oxidative stress under cold ischemic condition in vitro. Therefore, this study was designed to examine the effect of PRP on the MMP and apoptosis of MSCs after cold hypoxic injury.
Materials and Methods
Canine adipose-derived MSCs culture
Cells were provided from Animal and Plant Quarantine Agency, Korea. Low glucose Dulbecco’s modified eagle medium (DMEM-LG; Gibco, USA) containing 10% fetal bovine serum (FBS) (Gibco) and 139 µg/ml gentamycin was used as a culture medium. MSCs attached to the bottom of the plate and began proliferating in 2 days. The cells were washed with 0.05% Trypsin/EDTA-solution in sterile phosphate buffered saline (PBS) (Gibco). The plate was incubated for 3 minutes at 37℃, 5% CO2. Same volume of DMEM-LG containing 10% FBS and 139 µg/mL gentamycin was added. After centrifugation for 3 minutes at 2,000 rpm, 20℃, the MSCs were seeded at 10,000 to 20,000 cells/cm2 density and incubated in humidified 5% CO2 incubator at 37℃. The culture medium was replaced every 2 to 4 days. In this study, MSCs at passage 3 were used.
Preparation and activation of canine PRP
The blood was collected from the beagle dog and 40 mL of canine blood was used for 4 mL of PRP derivate. Ethical approval of animal studies was received from the ethical committee of Kyungpook National University (approval number: 2019-0088). Complete blood count test was performed to check for hematologic abnormalities. For manufacturing canine PRP, 2 PRP extracting kits (Goodmorningbio Inc., Korea) were utilized. Eighteen ml of blood with 2 mL of sodium citrate (C.T.G. Solution; Daehan New Pharm Co., Korea) was added in each kit. After collecting PRP kit containing canine blood was centrifugated for 5 minutes at 3,200 rpm at 20℃ (1580MGR; GYROZEN Co., Korea). After removing supernatant platelet-poor plasma, 2 mL of PRP was obtained from the kit. All of these works were performed under sterile conditions. PRP was collected into a conical tube and stored at -80℃ and thawed in water bath at 37℃ for 30 minutes just before use.
PRP treatment under cold ischemic condition
MSCs at passage 3 were each seeded in 24, and 96 clear-walled and 96 white-walled microplates (Corning, USA) (1 × 105 cells/well, 1 × 104 cells/well respectively) and incubated at 37℃, in humidified condition containing 5% CO2. At 80% confluency, cells were washed with sterile PBS and added 10% PRP in DMEM-LG. Only DMEM-LG was used as culture medium for serum-free control group. The plates were cultured in hypoxia incubator chamber (Stem cell Technologies Inc., Canada) under hypoxic condition with 1% O2, 5% CO2, and 94% N2 at 4℃ in humidified condition for 24 hours and 48 hours.
Evaluation
In this study, 4',6-diamidino-2-phenylindole (DAPI; Sigma Aldrich, Switzerland) immunofluorescence staining, MitoTracker Red CMXRos (Invitrogen, USA) staining, and caspase-3 activity measurement by Caspase-Glo 3/7 assay kit (Promega Co., USA) were used for assessment of the apoptotic changes of MSCs. Before the evaluation, MSCs at passage 3 (500,000 cells/mL) were seeded at appropriate multi-well microplates and incubated for 24 hours at 37℃ with humidified condition containing 5% CO2. The medium was removed, and non-treated and 10% PRP-treated media were added, incubated in the hypoxia chamber at 4℃ for 24 hours and 48 hours.
MMP assessment
Cell permeant MitoTracker Red CMXRos was used to assess the MMP of cells. To trace the intracellular exchange of mitochondria, cMSCs were labeled according to the manufacturer’s protocol. In brief, media were discarded from cMSCs-seeded 24 well multiplates and 96 clear well plates after 24 hours and 48 hours. The multiplates containing 0.1 µM final concentration of MitoTracker Red CMXRos dissolved in dimethyl sulfoxide were incubated for 30 minutes at 37℃ humidified condition containing 5% CO2. Cells in each 24, 96 well plates were washed and qualitative analysis were carried out by fluorescence microscopy and fluorescence microplate reader (Infinite 200 Pro; Tecan, Switzerland), respectively.
DAPI staining
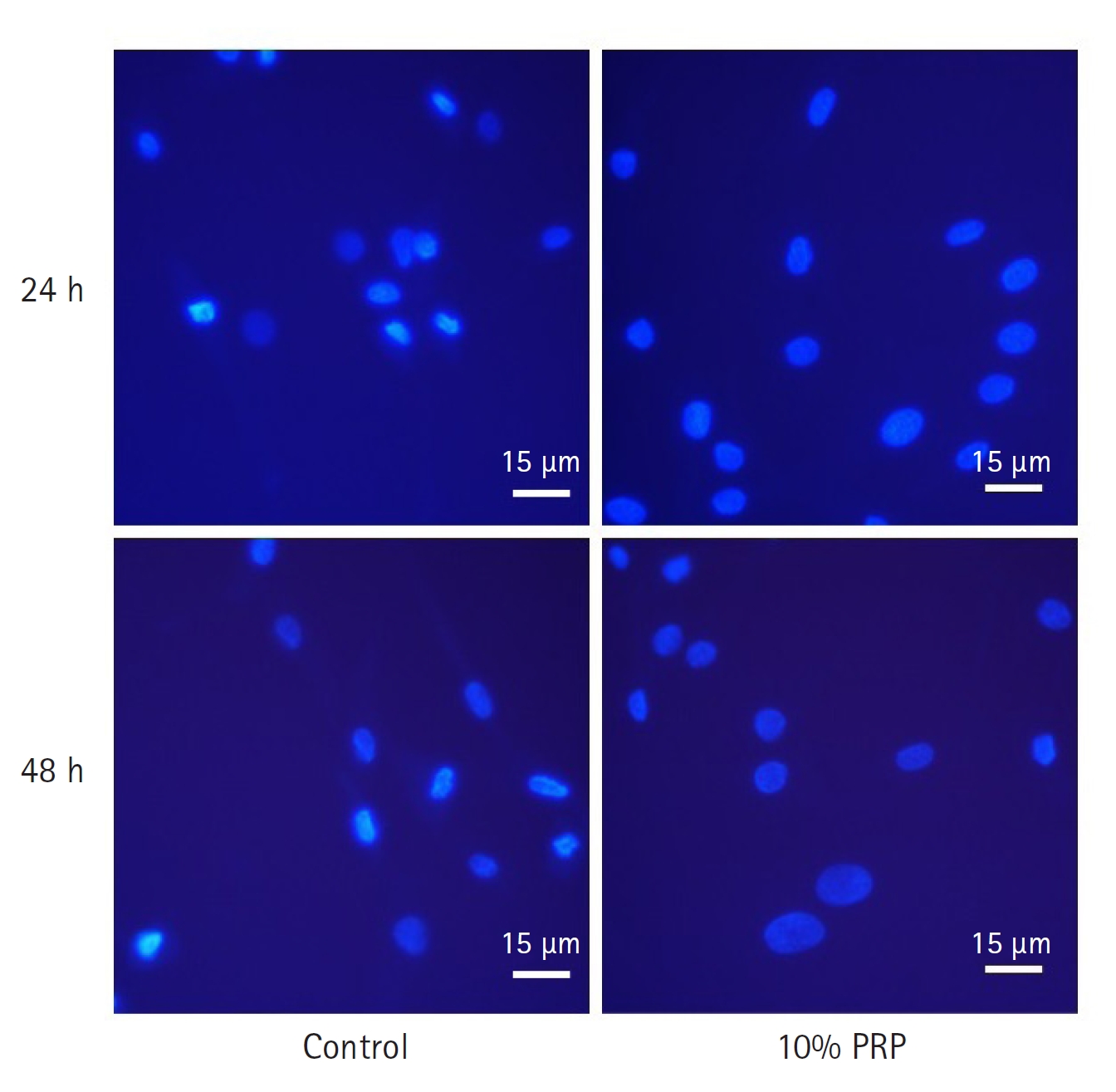
DAPI staining was used for obtaining immunofluorescence images, which shows condensation and fragmentation of cells followed by apoptosis. After removing the medium cells were fixed in 10% formaldehyde in PBS for 10 minutes at room temperature. After several washes, cMSCs were stained with 300 nM DAPI final working solution and placed at room temperature for 5 minutes, protected from light before observing fluorescence microscopy. All the cell numbers were counted in captured pictures at × 400 and the ratio of the numbers of apoptotic cells was recorded.
Caspase-3 activity assay
Caspase-3 activity assay performed by Caspase-Glo 3/7 assay kit was proceeded as the manufacturer’s protocol. White-walled 96 microplates were used, and the same amount of the reagent was added in each well without removing media. The plate was incubated for 2 hours at room temperature and the luminescence intensity assay was carried by luminometer (Infinite 200 Pro).
Statistical analysis
For statistical analysis, non-parametric Mann-Whitney U-tests were performed by Prism 8 (Systat Software Inc., USA). A value of p ≤ 0.05 was considered statistically significant. A p-value ≤ 0.001 was considered highly significant. All data are expressed as mean ± standard deviation.
Results
MMP assessment
Comparing with the control groups, the fluorescence intensity of 10% PRP groups were significantly higher at both hours. At 24 hours, the fluorescence intensity of the control group was 9,294.17 ± 99.21, that of the 10% PRP group was 9,605.50 ± 113.52. At 48 hours, the fluorescence intensity of the control group was 9,041.00 ± 73.71, that of the 10% PRP group was 9,334.00 ± 102.47 (p < 0.01, respectively) (Fig. 1). Cytoplasmic dye was apparent which means higher MMP in the 10% PRP groups at both hours (× 400) (Fig. 2).
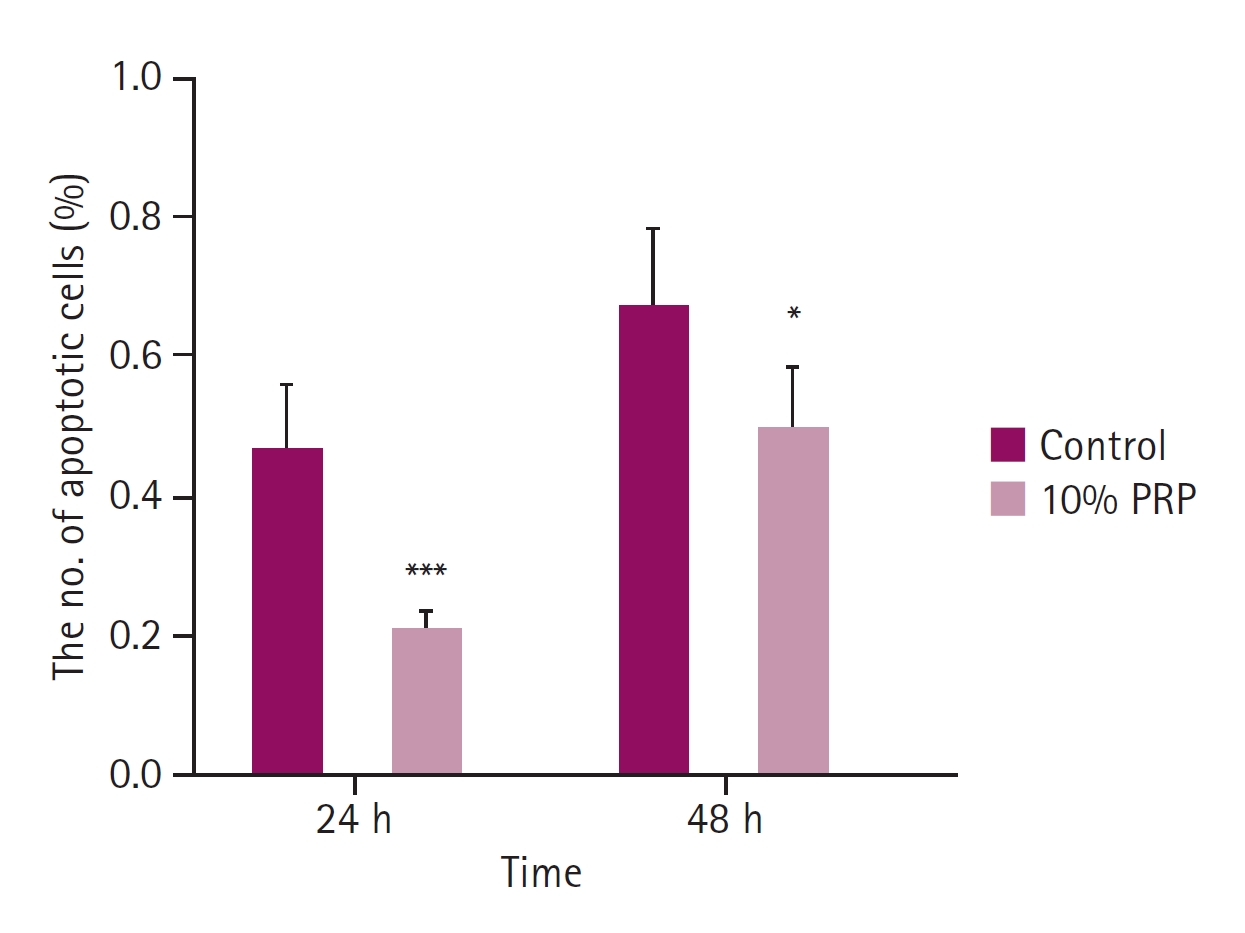
The ratio of apoptotic cells in 4',6-diamidino-2-phenylindole staining. The apoptotic changes of the control group were significantly higher than 10% platelet-rich plasma (PRP) group at 24 and 48 hours. Compared to 24 hours, the ratio of cell apoptosis was higher at 48 hours. *p < 0.05, ***p < 0.001.
DAPI staining
Comparing with the control group, the apoptosis of cMSCs was significantly less than 10% PRP treated group at 24 and 48 hours (p < 0.05, respectively). At 24 hours, the mean ratio of apoptotic cells was 0.47 ± 0.09, that of the 10% PRP group was 0.22 ± 0.03. At 48 hours, the mean ratio of the control group was 0.67 ± 0.12, that of the 10% PRP group was 0.50 ± 0.09 (Fig. 3). As shown on the Fig. 4, 10% PRP-treated MSCs showed less shrinkage and condensation of nuclei at both times.
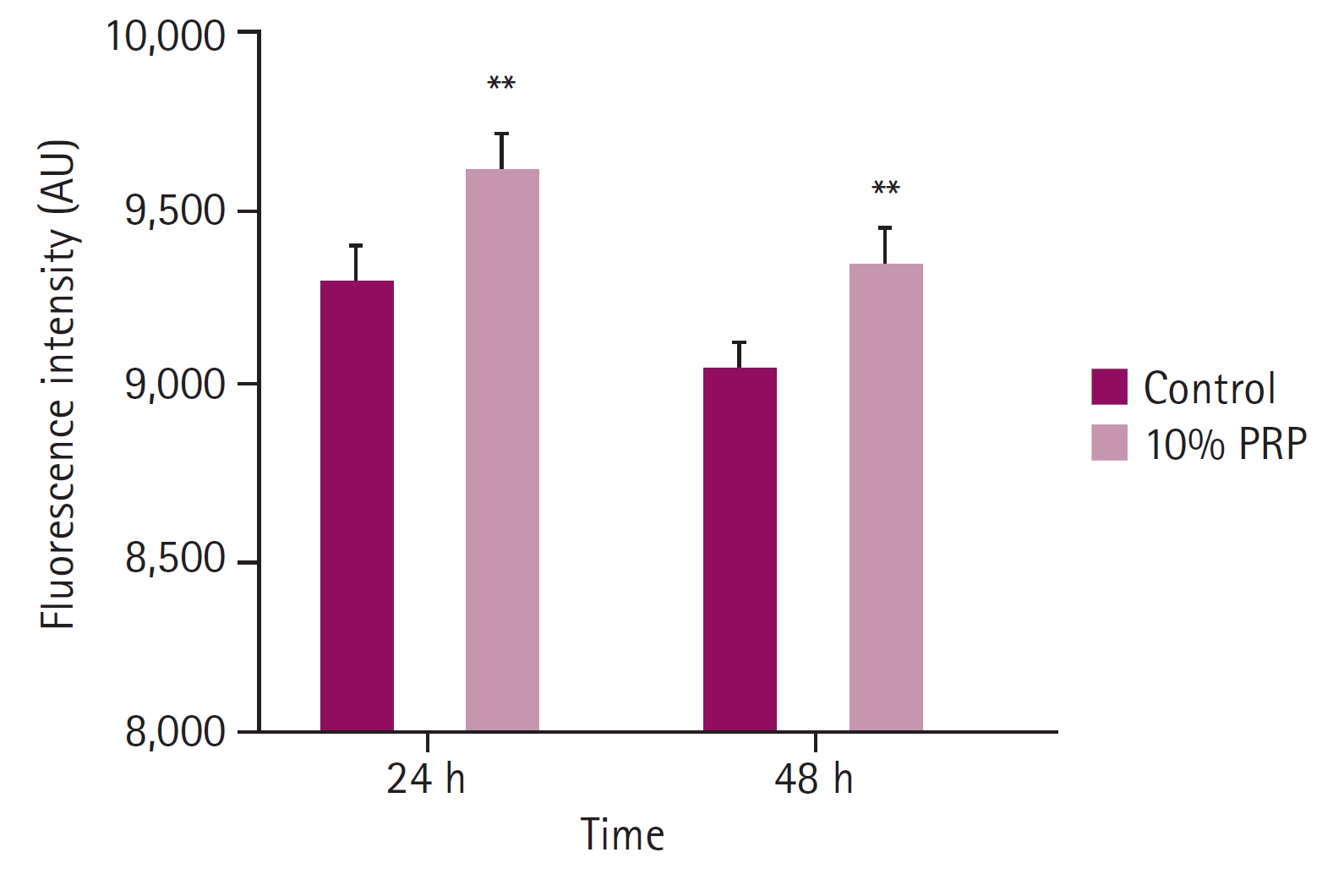
Fluorescence intensity of mitochondrial staining. Fluorescence intensity at 24 and 48 hours observed by microplate reader showed significant differences between control groups and 10% platelet-rich plasma (PRP)-treated groups. Fluorescence intensity of the cells at 48 hours decreased compared with that at 24 hours. **p < 0.01.
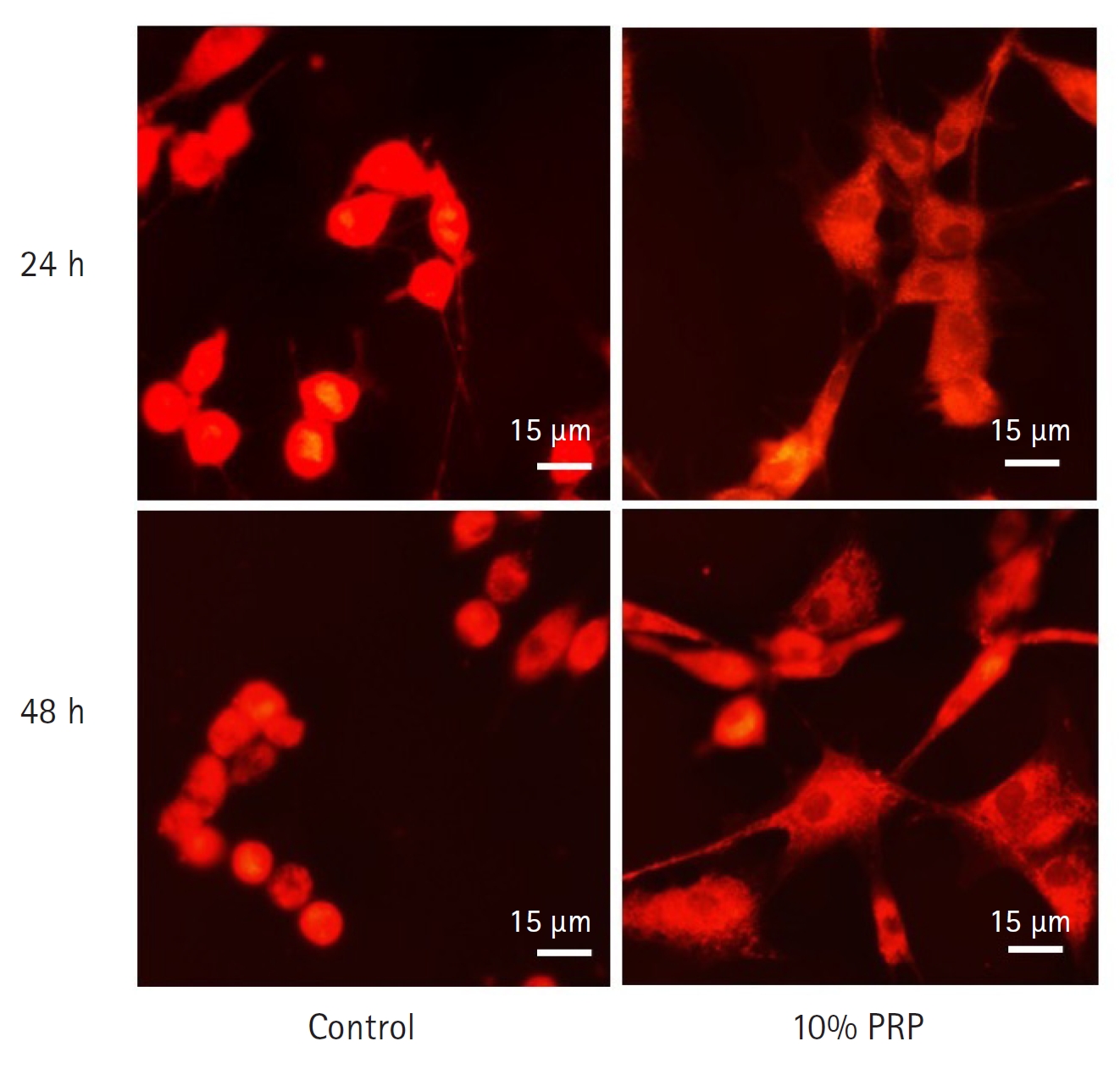
Mitochondrial staining by MitoTracker Red CMXRos (Invitrogen, USA). The red mitochondrial staining of 10% platelet-rich plasma (PRP)-treated groups at 24 and 48 hours were remarkable than control groups. Cytoplasmic staining was apparent on the PRP-treated group at 48 hours than those at 24 hours. Scale bar = 15 µm.
Caspase-3 activity assay
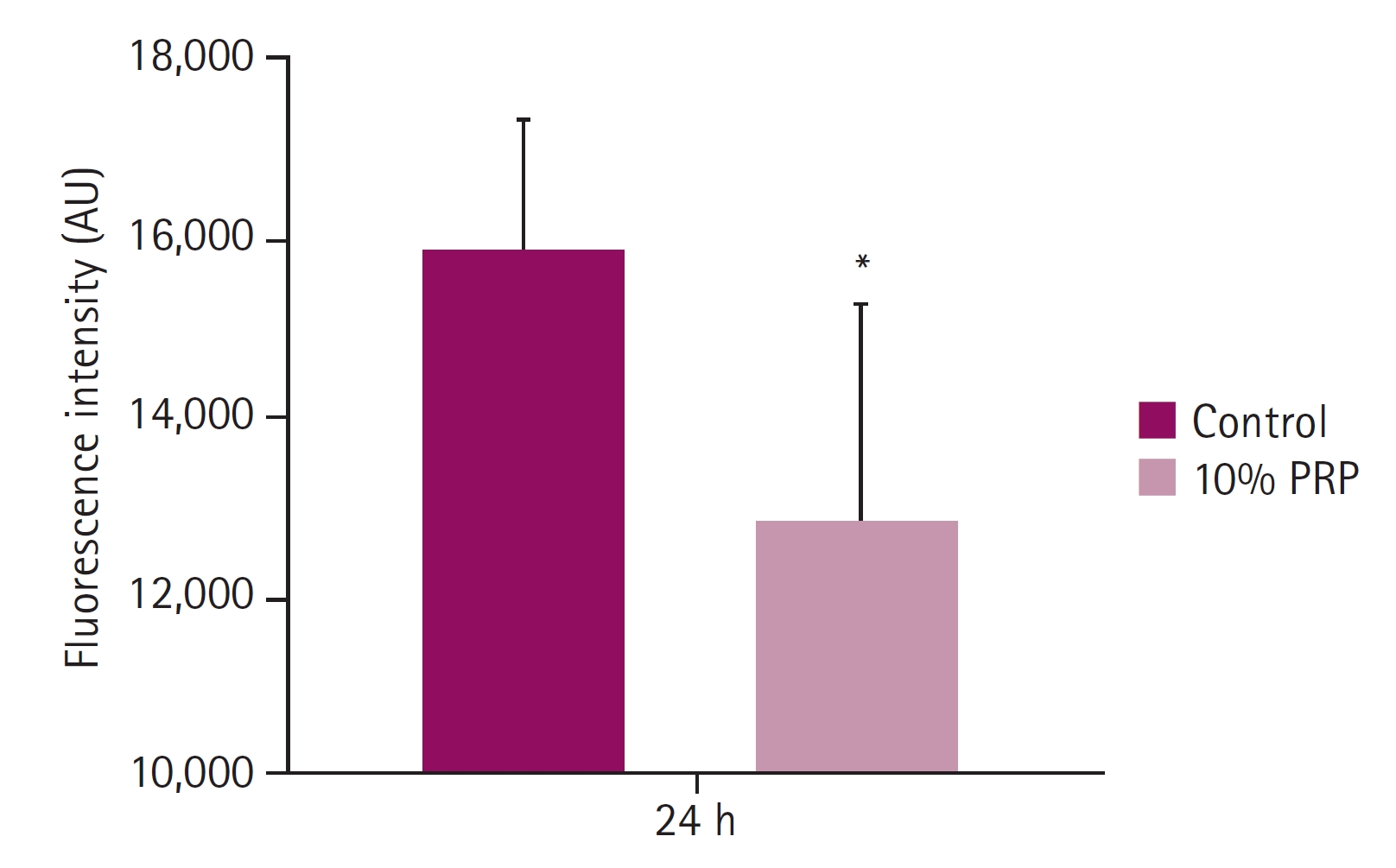
Compared with 10% PRP treated group, activation of caspase-3 of control group significantly increased. Luminescence intensity of the control group was 15,867.50 ± 1,443.36, that of 10% PRP group was 12,804.83 ± 2,416.11. There was a statistically significant difference (p < 0.05) between control and 10% PRP treated group after 24 hours (Fig. 5).
Discussion
In this study, it was examined that the apoptotic changes of cMSCs with canine PRP under cold ischemia, hypothesized that apoptosis would be prevented in PRP treated group. The apoptotic change of cMSCs treated with canine PRP groups were significantly decreased compared with non-treated groups at both 24 hours and 48 hours.
MSCs represent independent population of stem cells with self-renewal properties and have been presented to suppress inflammatory and immune-mediated responses by enzymes and cytokine secretions. MSCs are attractive candidates for therapeutic applications to repair or reconstruct damaged tissues, especially because these cells can be easily isolated from various sites from donor and proliferate. Especially, fat tissue is accessible and ubiquitous to harvest for getting multipotent stem cells with a minimal invasive procedure. Therefore, canine adipose-derived stem cells were used in this study.
There are several studies about O2 deprivation condition increasing cell death drastically [8,21]. Such external shocks generating oxidative stress may provoke amount of ROS and they induce cellular apoptosis [7], especially at cold ischemic condition. ROS are generated as a result of normal cellular metabolism and partly due to activation of oxidant-producing enzymes in response to exogenous stimuli [10]. However, overproduction or accumulation of ROS lowers MMPs and leads to the swelling and disruption of mitochondria, which is followed by cell death [11,12].
As mentioned above, apoptosis is the programmed cell death characterized by morphologic changes and energy-dependent biochemical modifications. In the mitochondria-dependent pathway, the executional cascade activating caspase-3 results in cytomorphological features including cell shrinkage, chromatin condensation, and nuclear fragmentation, etc. [15]. The cell disruption caused by various apoptotic stimuli shows collapse of MMP, breakdown of the outer mitochondrial membrane, and release of apoptotic protein like cytochrome c, which results in activation of caspase-3 and finally apoptotic cell death [15,18].
PRP is a blood-derived fraction containing high concentrations of platelets and growth factors [24,25]. PRP releasates also have an antiapoptotic effect to various kind of cells such as adipocytes, chondrocytes, and osteoblasts, which could make it available for broad application in clinical medicine [27]. In fact, it has been used widely in various therapies such as soft tissue healing, cartilage repair and bone regeneration [31,33]. PRP also has been used in ischemic injuries for cyto-protective role which ameliorate the oxidative stress and inhibit induced apoptosis. Based on that, this study was designed for observing antiapoptotic effect of canine PRP on cMSCs in cold ischemia which induces apoptosis much more than in normoxic condition.
Loss of MMP has been shown to release cytochrome c activating caspases and thus promote apoptosis. The reduction in MMP is considered to be the earliest event during the process of cell apoptosis, before characteristics of nuclear apoptosis appear (concentration of chromatin and break of DNA) [21]. In this study MitoTracker Red CMXRos dye was used as a measure of examining mitochondrial function by estimating the level of MMP.
DAPI staining showed the early stage of apoptotic changes, apparent nuclear condensation and fragmentation. There was marked nuclei shrinkage and fractionation in control groups for all hours. When the cells were dyed with MitoTracker, red mitochondrial staining of the 10% PRP treated groups showed fibroblast-like cell morphology and enhanced cytoplasm. Also, it was found that the fluorescence intensity of the PRP treated groups after cold storage was significantly higher than that of control groups, which is consistent with microscopic observation.
Compared to apoptosis, necrotic cells show swelling with chromatin condensation and eventually leading to cellular and nuclear lysis. In addition, divided apoptotic bodies at the end stage are surrounded by an intact plasma membrane. The DAPI-stained nuclei of control groups were condensed and fragmented into several pieces rather than lysis. Therefore, these results indicate that PRP protects mitochondrial disruption and that may have antiapoptotic effect under cold ischemic condition.
Caspase-3 is the executional factor of the apoptotic pathway. Apoptotic signals make procaspase-3 stimulated to become caspase-3. The activation of caspase-3 induces cellular apoptosis. In this study, the caspase-3 activity assay presented significant decrease in 10% PRP treated groups compared with control groups. Consistent with the result, D'Esposito et al. [40] observed that platelet derivatives reduce caspase-3 cleavage in adipose-derived MSCs. Furthermore, Kim and Park [6] demonstrated that caspase-9, generally followed by caspase-3 activation in mitochondrial pathway, can be activated by ROS without the involvement of cytochrome c release under hypoxia. In addition, activated caspase-9 induces cytochrome c release from mitochondria. Thus, oxidative stress-induced ROS remarkably increases apoptosis with the release of cytochrome c. Taken together, the cold ischemic condition brings about high caspase-3 activity leading to significant apoptotic change but PRP application may suppress the collapse.
There are some limitations of this study. First, the experimental groups that were used with only 2 conditions such as cold ischemic and PRP added cold ischemic states. Second, when planning to find the apoptosis conditions, the early and late apoptotic processes of cold ischemic conditions were not examined in the present study. For these reasons, it needs to be considered in future study that the anti-apoptotic effect of PRP will be observed through more various time points such as 12 hours or less and/or 72 hours or more.
In summary, increased cytoplasmic mitochondrial function and less apoptotic morphology were examined by DAPI staining and MMP assessment in PRP-treated groups. Caspase-3, the final activation factor during apoptotic pathway, was significantly lower in PRP-treated groups. These results present that PRP would act as an effective suppressor of cellular apoptosis. In conclusion, our data suggests that canine PRP has an antiapoptotic effect to cMSCs under cold ischemic conditions.
Notes
Conflict of interest
Seong-Mok Jeong is an associate editor of Korean Journal of Veterinary Research. The other authors declare no conflict of interest.
Funding
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (2021R1F1A1064483).
Data Availability Statement
Contact the corresponding author for data availability.
Author’s Contributions
Conceptualizaion: Kwon YS, Jeong SM; Data curation: Shin S; Formal analysis: Shin S; Funding acquisition: Kwon YS; Investigation: Kim SE; Methodology: Shin S, Kwon YS; Project administration: Kwon YS; Resources: An SW; Software: Kim SE; Supervision: Kwon YS; Validation: Kim SE; Visualization: Shin S; Writing–original draft: Shin S, Kim SE; Writing–review & editing: An SW, Jeong SM, Kwon YS.