Introduction
Dirofilaria immitis, called canine heartworm, is a vector-borne disease, and it is a parasitic roundworm that causes dirofilariasis. It is spread from host to host through the bites of mosquitoes, and it inhabits the right ventricle and pulmonary arteries of canines. Hosts diagnosed with heartworm infection may have serious complications, and the disease typically culminates in death after secondary congestive heart failure. The definitive host is the dog, but it can also infect other animals such as cats, wolves, coyotes, foxes, and, under rare circumstances, humans [1].
The Companion Animal Parasite Council in the U.S. has reported that over 100,000 dogs are diagnosed with heartworm infection annually [2]. In South Korea, 25.1% of households have pets, and 75.3% of those have dogs [3]. Changes in perceptions of pets have occurred in recent years in South Korea, but dogs have traditionally been recognized as guards, so the majority of dogs live outside. In addition, as the popularity of pets increases, the number of dogs abandoned is increasing year by year. These outside dogs, rescue dogs, and dogs that become wild dogs after being abandoned are exposed to heartworm infections. Climate and weather conditions affect the vector ecology and dogs’ exposure to infection [4]. In the last 30 years, temperatures have increased 1.4°C, and the highest average annual temperature was 14.1°C in the 2010s (2011-2017). Precipitation in the last 30 years has increased 124 mm. In this period, summer has become 19 days longer, and winter has become 18 days shorter [5]. The ongoing trends of increasing temperature and more variable weather affect the dog population at risk of infection.
Prediction of the population of infected dogs depending on the vector (mosquito) population can serve as a blueprint for the prevention of diseases such as quantifying of drug preparation. Mathematical modeling has become an important measure for analyzing the epidemiological characteristics of infectious diseases. These epidemiological models provide the population that remains in the absence of disease, called a disease-free equilibrium (DFE) [6]. Coyne et al. [7] proposed a susceptible, exposed, infected, and recovered (SEIR) model, and one study described the population dynamics of a rabies epidemic in raccoons using the SEIR model. Moreover, transmission pathways of mosquito-borne disease dynamics for humans have been actively analyzed through mathematical approaches [8,9]. However, canine heartworm infection transmission has not been reported yet through mathematical modeling.
Materials and Methods
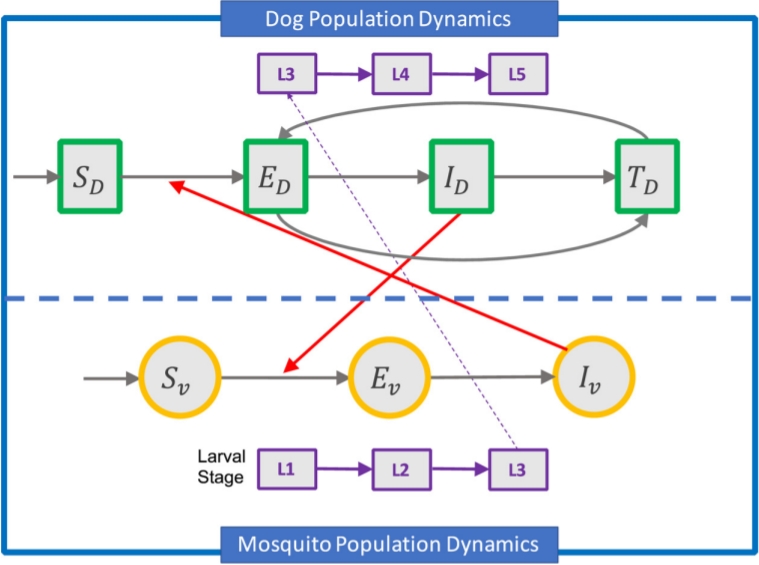
Heartworms can be transmitted from dog to dog by mosquitoes. Microfilariae (young heartworm) enter the system of the mosquito when an infected dog is bitten by a mosquito, called the first larval stage (L1). In the second larval stage (L2), the microfilariae develop into infective larvae inside the mosquito within two weeks, and in the infective third larval stage (L3), they can be transmitted to other dogs. After 1-2 weeks, they enter the fourth larval stage (L4). Then, they migrate to the muscles of the chest and abdomen, entering the fifth stage (L5). It takes approximately 6 months for the infective larvae to mature into adult heartworms, which is known as the “prepatent period.” During the first 3 months, the larvae migrate through the dog’s body, eventually reaching the blood vessels of the lung, and during the last 3 months, the immature worms grow to adults to lengths of up to 35 cm. The blood vessels are destroyed by the worms, which causes severe lung and heart disease. When the worms mature, the adult worms mate and produce new microfilariae, which can cause damage to the other organs of dogs. This lifecycle continues and repeats when a mosquito bites an infected animal, becoming infected by the microfilariae. Adult heartworms can survive in dogs for 5 to 7 years [10]. To determine the dynamic transmission route of canine heartworm infection, we utilize a system of nonlinear ordinary differential equations (ODEs) as the deterministic mathematical model. We consider two subpopulation transmission models of canine heartworm in dogs: dogs and mosquitoes. The dog population has four categories: susceptible dogs SD, exposed dogs ED infected dogs ID, and partially immune dogs RD. The total dog population is denoted by ND = SD + ED + ID + RD. Mosquitoes acting as the vector are categorized into susceptible mosquitoes Sv, exposed mosquitoes Ev, and infected mosquitoes Iv. The total number of mosquitoes is given by Nv = Sv + Ev + Iv. Recovered mosquitoes are not considered in this model. The list of the state variables used in this model is shown in Table 1. A schematic of heartworm infection transmission is illustrated in Fig. 1.
The model is a system of ODE as follows:
All parameter values are shown in Table 2. Since a mathematical model of heartworm infection has never been studied, we assume parameters of mosquito biting rate b, incubation rate in mosquitoes δv, and natural mortality rate of mosquitoes μ from other research in Zika dynamics or rabies. Recruitment rates of dogs α(N) are estimated from recent (2017) data from South Korea [11]. βvD, the infected mosquito-to-dog transmission rate, is taken as the same value as the infected mosquito-to-human transmission rate in [8]. The incubation rate in dogs is approximated to 0.005(days-1), since the prepatent period is normally 4-7 months [10]. The system of ODE as shown in Equation (1) was implemented in MATLAB, and the code is available at https://github.com/venusseo12/Mathematical_Modeling_of_Dirofilaria_immitis.git.
Results
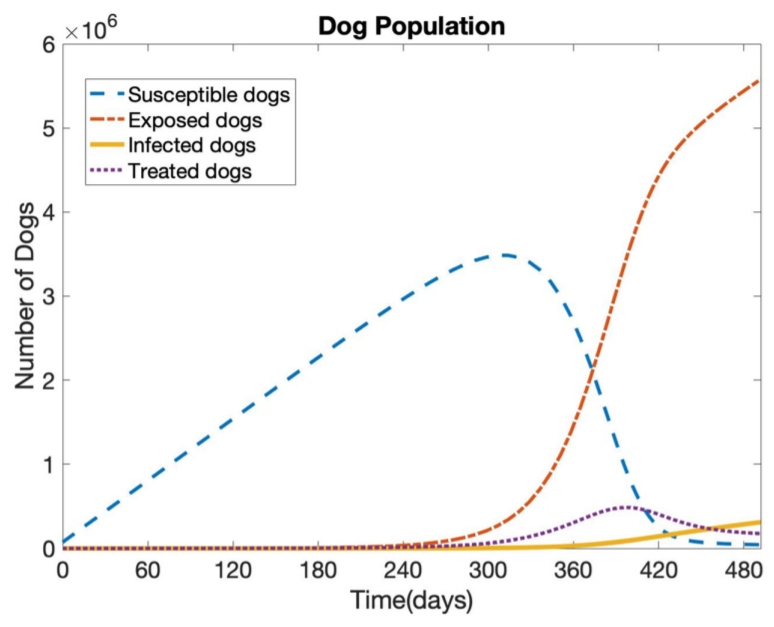
To understand the transmission pathways on the heartworm transmission dynamics between dogs and mosquitoes, we construct the model as a system of ODE, as shown in (1). MATLAB is used to obtain the numerical results, which are the different stage populations of dogs and mosquitoes (susceptible, exposed, infected, and treated). We do not consider the final stage, treated, for mosquitoes. We take all parameter values as shown in Table 2 for the base model. Fig. 1 shows the simulation results that represent the four different stages (susceptible, exposed, infected, and treated) of the dog population without control for 1 year. We assume that the number of recruited dogs (homeless, sheltered, or outdoor dogs) is 73,602 [11]. Simulation results show that after 1 year, 3,289 dogs out of 73,602 (about 4.5%) are exposed and 134 dogs are infected, as shown in Fig. 2. Only 0.2% of susceptible dogs become infected after 1 year, which seems low. However, this is because it takes about 6 months for exposed dogs to be infected through the procedure of the young worms maturing into adults and then migrating through the dog’s lungs and other organs. Thus, if all exposed dogs are maintained in the same circumstances without any treatment, then the number of infected subjects will increase over time. This may increase the possibility of other dogs, especially dogs that live outside, being infected.
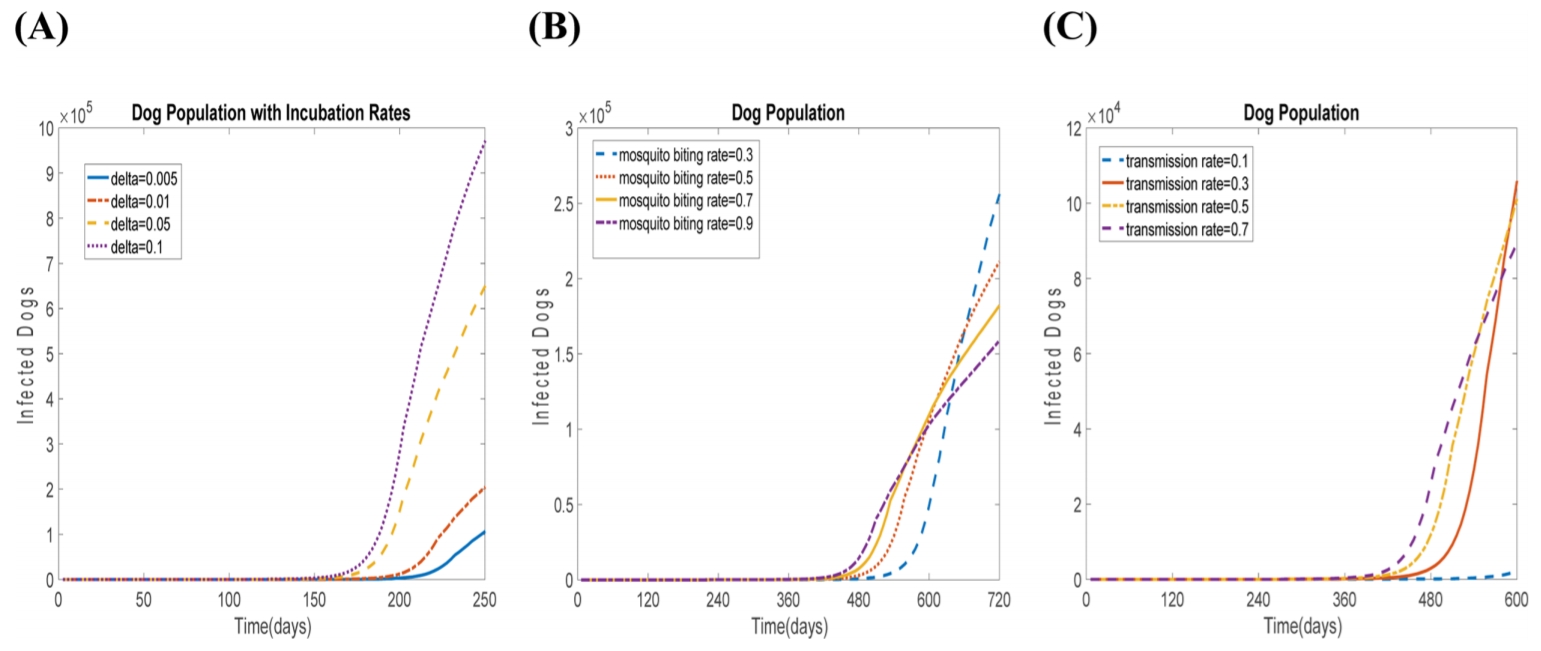
In order to estimate the accuracy of the parameters, we also experiment numerically with various values of incubation rate, mosquito biting rate, and infected dog-to-mosquito (or vice versa) transmission rate (see Fig. 3). As the mosquito biting rate is higher, the number of infected dogs also increases in the first 600 days. However, the growth rate of the mosquito biting rate 0.3 is the highest after 600 days. This implies that the parameter of mosquito biting rate is not significantly important for infection in the long term. Fig. 3C shows the infected dog population with different infected dog-to-mosquito transmission rates. The value of 0.1 is not applicable for our simulation, as shown in Fig. 3 (C, bluedashed line).
Discussion
We created a mathematical model of heartworm infection transmission dynamics in dogs and mosquitoes. Mathematical models for infectious disease dynamics have been used for a variety of diseases, and they suggest problems to solve for disease-free environments. Vector-borne diseases whose vector is the mosquito, such as Zika and Dengue, are especially actively studied by academic institutes. However, epidemic models with the dog as the host have rarely been studied. Some African and Chinese research teams have suggested models of rabies related to transmission from dogs to human [14,15]. To date, research with mathematical epidemic modeling has focused on diseases related to human beings. In the past, the number of dogs in South Korea and the number of dogs with diseases were not officially reported. It is expected that research on these pets will be actively conducted by the national dog registration system in South Korea. Prediction of the population of infected dogs depends on the vector (mosquito) population, and it will serve as a blueprint for the prevention of diseases as well as quantifying of drug preparation. We have some goals for future studies to develop the best model of heartworm infection transmission dynamics that predicts the optimal way of preventing deaths among dogs. First, we will continue to perform stability analyses to determine the locally and globally asymptotically stable DFE. Second, we will create a model with respect to the death rate, birth rate, and other parameter estimations for dogs and mosquitoes. It is desirable to estimate the effective and optimal use of vaccinations in dogs for eliminating the disease in South Korea.


 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print