Bovine tuberculosis (bTB) is a contagious and chronic zoonotic disease caused by
Mycobacterium bovis. Globally, bTB is reported to occur in various hosts, such as cattle, deer, dog, cat, otter, and humans [
1]. However, in the Republic of Korea, it has only been reported in cattle, deer, and otters [
2]; it is transmitted via direct and indirect contact with infected animals, and its clinical signs include weakness, weight loss, intermittent hacking cough, diarrhea, and anorexia, among others [
3]. Many countries have implemented preventive and control policies, such as diagnostic testing and slaughter of infected animals, vaccination, pasteurization of milk, and monitoring of wild animals, to eradicate this disease [
4]. In the Republic of Korea, cattle and deer are regularly screened for bTB infection, and infected animals are culled. In addition, only bTB-negative animals are transported to farms or slaughterhouses.
Pasteurellosis, caused by
Pasteurella multocida is a major bacterial disease in domestic rabbits [
5]. It is transmitted primarily via direct contact, although transmission via indirect contact with infectious aerosol and with the secretions and exudates of infected vectors may also occur.
P. multocida is a normal microflora in the oral cavity or upper airways of animals; however, when the host is immunocompromised due to malnutrition or stress consequent to dense breeding, it causes pathogenic infections. Pasteurellosis shows various clinical signs, such as rhinitis, pneumonia, otitis media, endometritis, orchitis, abscess, and septicemia [
5].
To our knowledge, unlike pasteurellosis, bTB has rarely been reported in domestic rabbits worldwide, including the Republic of Korea. In this report, we describe the pathological findings of a rare case of bTB infection in a domestic rabbit.
On March 22, 2019, a deceased 12-month-old domestic rabbit was brought to the Animal and Plant Quarantine Agency (APQA; Korea) to identify the cause of mortality. It died suddenly without manifesting specific clinical signs, except for discharge from the eyes. The habitat was a farm breeding 100 rabbits in Yecheon, Gyeongsangbuk-do province, Republic of Korea.
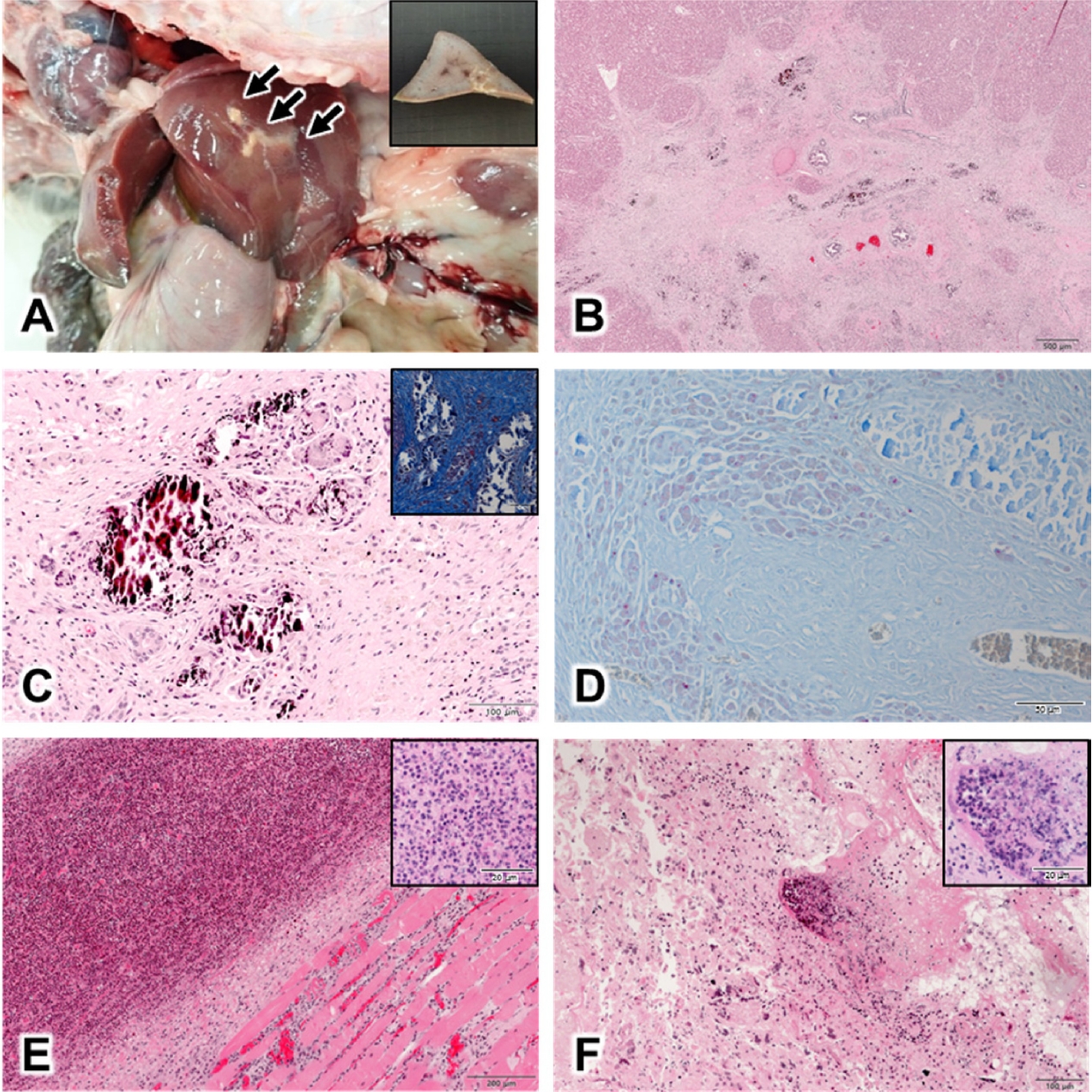
Gross visualization at necropsy showed irregular yellow nodules on the liver (
Fig. 1A), and multiple pus in the submandibular muscles, lungs, uterus, and small intestines. For histopathological exanimation, all organs, including the liver were fixed in 10% neutral buffered formalin for 24 h and were processed routinely. Finally, the formalin-fixed tissues were sectioned and stained with hematoxylin-eosin and Ziehl-Neelsen stain [
2]. Histopathological examination showed that most lesions were round or oval, and sometimes polymorphic in the liver. Mineralization was found in the center of granulomas, and were scattered all over the lesion. The hepatic nodules were surrounded by proliferative connective tissue indicative of granulomatous inflammation, and demonstrated mineralization; epithelioid macrophages and Langerhans-type giant cells were also observed (
Fig. 1B,
1C), in addition to pink acid-fast-positive bacteria (
Fig. 1D). Additionally, suppurative inflammatory lesions were present in the submandibular muscles, lungs, uterus, and small intestines; microscopically, these were composed of aggregated neutrophils, lymphocytes, and necrotic cell debris (
Fig. 1E,
1F).
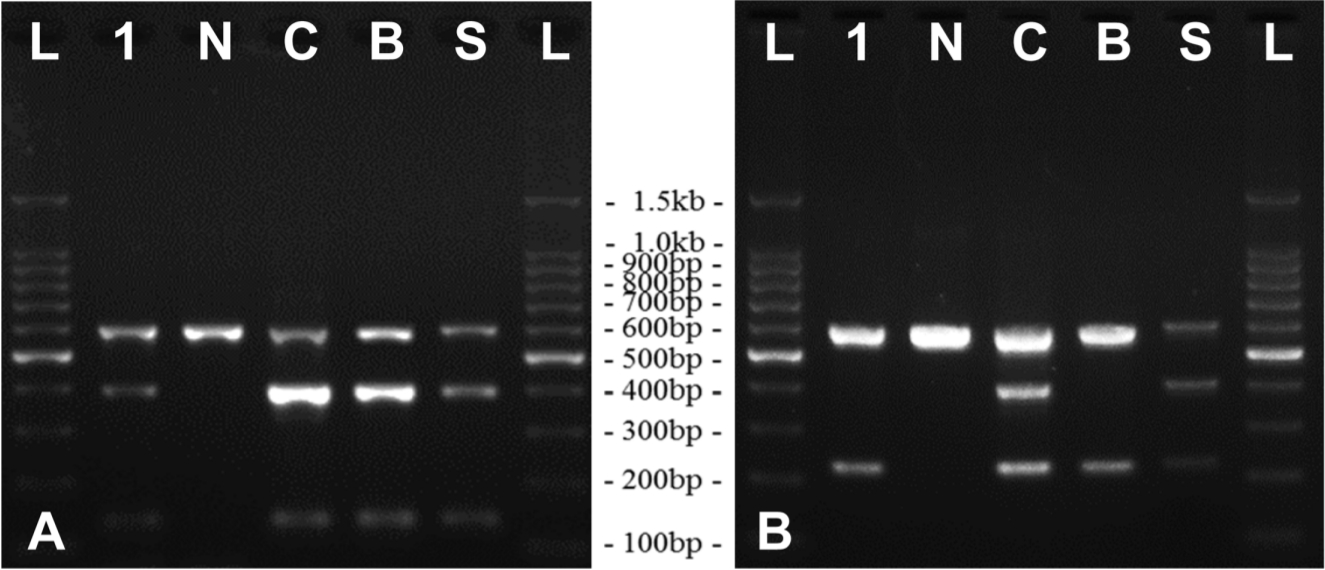
After gross and histopathological examination, the differential diagnosis was performed based on bacterial identification. The lesions in the tissues were inoculated in 5% sheep blood agar (Asan Pharm. Co., Korea) and MacConkey agar (Becton Dickinson, USA), and pathogen-specific genes were amplified by polymerase chain reaction (PCR). Automated genomic DNA extraction was performed with Maxwell
┬« RSC 48 (Promega, USA), according to the manufacturerŌĆÖs instructions, from homogenates of various tissues including the liver, which had nodules and were suspected of tubercle. In particular, DNA extracted from the liver was used in the PCR assay for the detection of
Mycobacterium tuberculosis complex, including
M. tuberculosis and
M. bovis. IS1081 and IS6110 regions, insertion elements in the
M. tuberculosis complex genomes [
6,
7], were amplified using the DiaPlexC
TM MTB detection kit (Solgent, Korea). PCR amplification of liver DNA was positive for specific genes of
M. tuberculosis complex (
Fig. 2A). Differentiation of
M. tuberculosis and
M. bovis DNA was performed using the DiaPlexC
TM MTB/
M. bovis detection kit (Solgent, Korea), which showed specific amplicons of
M. bovis (
Fig. 2B). Isolation of
Mycobacterium spp. was attempted to identify the bacteria, and for further analysis, as described previously [
8]. However,
M. bovis was not isolated owing to the presence of various microbial contaminants. The differential diagnosis was performed from the suppurative foci of multiple internal organs. Suspected enriched mucoidal and non-hemolytic colonies were subjected to biochemical identification using the VITEK II system (BioM├®rieux, France), and bacterial identification was confirmed with species-specific
kmt1 amplification [
9,
10].
P. multocida was identified in the suppurative lesions.
Spacer oligonucleotide typing (Spoligotyping) was performed to determine the Mycobacterial species, and to identify its genotype. Spoligotype pattern was analyzed using the REBA MTB-Spoligotyping
® kit (Optipharm M&D, Korea), and was deposited in the
M. bovis spoligotype database (
http://www.mbovis.org) for comparison with various previously documented Mycobacterial spoligotype patterns [
11,
12]. The results indicated that the
Mycobacterium spp. in the liver nodules was of spoligotype SB1040, previously determined in
M. bovis.
Pathological features, amplification of M. tuberculosis complex-specific genes, and the spoligotype supported a diagnosis of bTB infection due to M. bovis. Finally, this case was diagnosed as a case of comorbid pasteurellosis and bTB.
Most bTB cases show lesions in various organs, including the respiratory tract and lymph nodes; however, in the present case, it was confined to the liver. This was considered to have occurred consequent to extrapulmonary dissemination, which is known as a particularly common transmission in rabbits infected by
M. bovis [
13]. The mycobacterial infection in this case was assumed to have been caused by the breeding environment. The rabbits were bred in burrows in bare ground, similar to wild rabbit warrens. However, as the environment has been considered to be a pathogenic reservoir [
14], rabbits in this study were potentially vulnerable to infectious diseases. In this case, it was presumed that the rabbit was infected through contact with infected bodies or soil in the burrow. In addition, bovine tuberculin purified protein derivative (PPD) inoculation tests in the co-habiting rabbits from this farm were negative in all cases (data not shown). These results therefore, indicate that the possibility of air transmission is very low. However, further studies considering the epidemiological situation, is required for elucidating the path of infection.
In contrast, the pasteurellosis lesions were acute, systemic, and typical. In this case, the cause of death was assumed to be pasteurellosis caused by P. multocida, and not bTB by M. bovis. P. multocida was thought to invade acutely and systemically by an opportunistic infection into the host rabbit, which may have been immunocompromised because of chronic bTB. In addition, the cause of death in 6 co-habiting rabbits was identified as pasteurellosis with suppurative inflammations in their submandibular muscles, lungs, uterus, and small intestines; this further supported the spread of P. multocida infection.
On the basis of the Korea Animal Health Integrated System (KAHIS;
http://www.kahis.go.kr), the region with mostreported bTB cases among domestic animals in the last 10 years (2010-2019) was Gyeongsangbuk-do province, which accounted for 11 of the 27 cases reported. The farm in the present report was located in this province. In addition, SB1040 has been consistently reported as a major spoligotype pattern of
M. bovis for bTB cases in the Republic of Korea, along with SB0140, which was reported between 2012-2015 [
8]. Therefore, this case was considered as a natural outbreak of bTB in the deceased rabbit. However, the correlation between the bTB outbreak and farm location was difficult to establish owing to a lack of epidemiological information.
bTB has been rarely reported in wild and domestic rabbits, although laboratory rabbits demonstrate susceptibility to experimental infection with
M. bovis. Few isolated cases of
M. bovis infection in rabbits have been reported in several countries, such as the United Kingdom and New Zealand [
1]. Most previous studies have rarely reported the isolation of
M. bovis from rabbits.
M. bovis was not isolated from 146 dead domestic rabbits in the United Kingdom between 1976-1979, and from about 1,200 dead wild rabbits in New Zealand in 1991 [
1,
15]. Based on the data by KAHIS, particularly, most cases were reported for cattle, and several cases have been reported in deer and otters in the Republic of Korea. As tuberculosis was not detected in domestic rabbits, they have been excluded from the list of target animals for national surveillance. This case suggests that rabbits need to be considered as a susceptible host and reservoir for
M. bovis. In countries where bTB is prevalent, various potential hosts should be monitored for bTB infection.











 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



