Chronic hypertrophic pyloric gastropathy is common in small, purebred, middle- to old-aged dogs [
1,
2]. Hypertrophic gastropathy is described as a condition of unknown origin but associated with
Helicobacter pylori in 90% of patients in human medicine [
3]. The breeds included Maltese, Shih-Tzu, Yorkshire Terrier, and Chihuahua with mean age at presentation of 8.8 years, ranging from 3 to 15 years [
1,
2]. Approximately twice as many male as female dogs are affected [
1], presenting with clinical signs such as chronic vomiting, weight loss, polydipsia, depression, lethargy, anorexia, and abdominal pain [
2]. Definitive diagnosis of chronic hypertrophic pyloric gastropathy requires a full-layer biopsy of the stomach, and diagnostic imaging can aid the diagnosis of chronic hypertrophic pyloric gastropathy [
1]. But diagnostic imaging reports of hypertrophic pyloric gastropathy are rare, and computed tomography (CT) reports only one case. This report describes a diagnostic imaging and treatment of hypertrophic pyloric gastropathy with
Helicobacter spp. in a dog.
A 12-year-old, castrated male, mixed dog weighing 4.85 kg was referred with a history of gradual abdominal distention for a year and anorexia recently. During physical examination, no significant findings were found except abdominal distention with serum chemistry showing hypochloremia (99 mmol/L, reference range 102 to 117 mmol/L).
Abdominal radiography showed a gastric distention with fluid (
Fig. 1A and
B). Thoracic radiography showed no significant findings. Abdominal ultrasonography revealed a solitary, pedunculated, heterogeneous mass (24.4 × 24.5 mm) arising from the mucosal layer and protruding within the lumen of the pylorus with intact wall layers. An evenly thick hypoechoic muscular layer (4 mm) was evident (
Fig. 1C and
D).
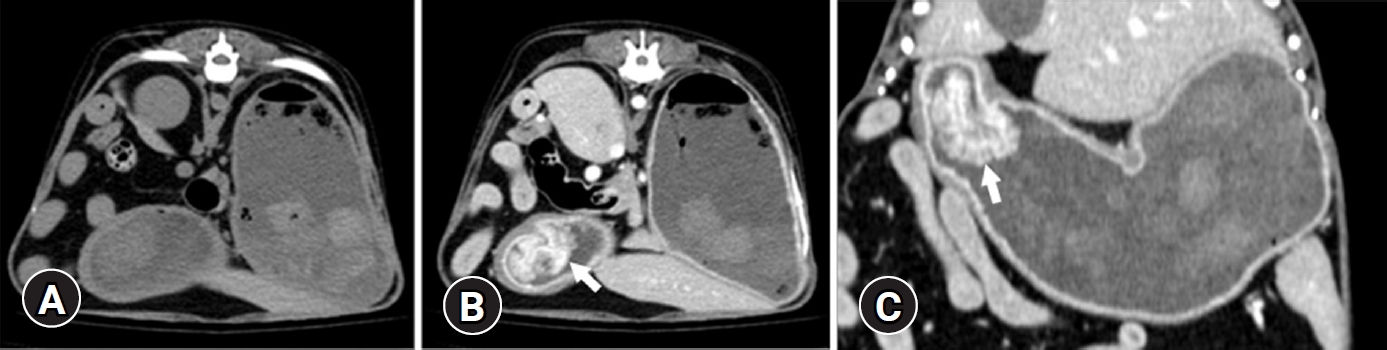
CT examination was performed using 32-multislice CT (Revolution; GE Healthcare, United Kingdom). The patient was positioned in ventral recumbency on the CT table under general anesthesia, having scanning parameters of 120 kV, 85 mA, and 0.6 mm slice thickness. Contrast study was performed after intravenous administration of 600 mgI/kg iohexol (Bonorex 300 Inj.; Daehan Pharm, Korea) injected for 20 seconds using autoinjector (A-60; Nemoto Kyorindo Co., Japan), obtaining delayed phase postcontrast CT images 90 seconds after injection. A narrow-based, polypoid mass (13.8 × 23.3 × 28.9 mm) in craniodorsal pyloric wall with remarkable homogeneous contrast enhancement and normal wall layers was detected (
Fig. 2). A pyloric canal was narrowed (3 mm diameter on the sagittal plane). A splenic lymph node was round and enlarged (6.7 × 9.9 mm) with normal contrast enhancement and a cyst-like component. Muscular layer hypertrophy in the pylorus and duodenum was identified. Based on radiography, ultrasound, and CT findings, differential diagnosis includes chronic hypertrophic pyloric gastropathy, gastric polyps, granulomatous gastritis, and less likely a gastric tumor.
After surgical excision of the mass, hypertrophic pyloric gastropathy with polypoid growth was histopathologically confirmed based on cystic hyperplasia of the gastric mucosal epithelium and infiltration of chronic-active inflammatory cells. Histological examination identified helical-shaped bacteria,
Helicobacter spp., infiltrating the gastric mucosal epithelium (
Fig. 3). After the 11-day follow-up, the dog was in good condition with no surgery complication.
Hypertrophic pyloric gastropathy causes the obstruction of the pyloric lumen or restriction of the luminal dilation and other conditions such as gastric polyps, granulomatous gastritis, tumors, pyloric foreign body, and intussusception [
4]. These diseases’ nonspecific clinical signs include vomiting, pain, and distention of the upper abdomen usually after a meal [
5]. Nonobstructive hypertrophic pyloric gastropathy can be subclinical, when the pyloric canal is sufficiently patent, allowing gastric contents to empty into the duodenum despite being thickened [
6]. Radiological findings are nonspecific for pyloric obstruction induced by hypertrophic pyloric gastropathy but can reveal a distended stomach with fluid or gas in the case of delayed gastric emptying [
1,
5]. The patient in the present study was considered to be nonobstructive hypertrophic pyloric gastropathy with nonspecific radiographic signs including mild gastric distension.
Ultrasound and CT are useful for differentiating the underlying disease for pyloric obstruction and grading the hypertrophic pyloric gastropathy [
1,
5]. In a previous study, hypertrophic pyloric gastropathy can be graded according to the distribution of gastric layers on histopathology and identified correlation histopathological findings with ultrasound [
1]. Grade 1 is characterized by a thickened muscular layer, while grade 2 has both mucosal hyperplasia and muscular hypertrophy [
1]. Grade 3 is characterized by mucosal hyperplasia with glandular and foveolar hyperplasia, glandular cystic dilatation, and mucosal/submucosal inflammation [
1]. The classification system has a bearing on the appropriate surgical management of the condition [
1]. In the same study, dogs with grades 1 or 2 show at least a 4-mm-thick muscular layer in the pyloric area on ultrasonography [
1]. This study shows hypoechoic pyloric muscular layer thickness of 4 mm and confirms cystic hyperplasia of the gastric mucosal epithelium and chronic inflammatory cells through ultrasound and histopathology, respectively. The results show that the present case is a grade 3 hyperplastic pyloric gastropathy.
While the CT images of hypertrophic pyloric gastropathy of the previous study revealed a homogeneous contrast enhancement of the thickened gastric wall [
5], the CT images of the present study showed a similar aspect of the contrast enhancement of the thickened gastric wall with intact gastric wall layering. However, our case showed mild enlarged splenic lymph node, whereas other previous studies revealed inflammation, such as giant hypertrophic gastritis with an enlarged gastric lymph node [
7,
8]. Similarly, the present study considered splenic reactive lymphadenopathy due to chronic inflammation.
Similar with hypertrophic pyloric gastropathy, larger neoplasia within the pylorus, such as adenocarcinomas and squamous cell carcinoma, can induce outflow obstruction [
4,
9]. The common ultrasound features of neoplasia include thickening of the stomach wall, distortion of the normal layering, and altered echogenicity and motility in the affected area [
4]. Metastasis to the regional lymph node can be detected. Cytology or histopathology is required to differentiate inflammation from neoplastic condition and to categorize the tumor type [
4]. On ultrasound and CT examination, intact gastric wall layer identification is important to differentiate hypertrophic pyloric gastropathy and gastric tumor [
4,
5]. On CT images, gastric tumor shows heterogeneous contrast enhancement, while hypertrophic pyloric gastropathy is homogeneously contrast enhanced like in the present study [
5].
Histopathology in the present study confirms the presence of multiple helical-shaped bacteria infiltrating the gastric mucosal epithelium and chronic inflammatory cells, indicating
Helicobacter spp. infiltration [
10].
Helicobacter spp. have been identified as one of the most common causes of gastritis and peptic ulcer, although its etiology is not completely elucidated [
10].
Helicobacter infection can stimulate increased release of gastrin and then induce acid secretion, causing gastric ulceration [
11,
12].
H. pylori infection induces hypertrophy of the gastric mucosa represented as a giant fold gastritis, by increasing apoptosis of surface and proliferative cells [
13-
15]; expands the proliferative cells; and affects the proliferative zone to the deeper gland as a compensatory response [
13-
15]. The correlation of
Helicobacter spp. with the cellular proliferative activity and potential phenotypic alterations in canine spontaneous gastric polyps was investigated [
13]. The presence of
H. pylori seems to induce overexpression of cyclooxygenase-2 in the deeper glands of gastric polyps, leading to an increased expression of this enzyme through the production of proinflammatory cytokines [
13].
H. salomonis, H. felis, H. bizzozeronii, and
H. heilmannii sensu stricto are the predominant gastric
Helicobacter spp. in cats and dogs [
14]. These non-
H. pylori Helicobacter species have significant correlations with mild to moderate epithelial injury and mild to moderate intraepithelial lymphocyte infiltration of the canine stomach [
14]. Based on previous studies,
Helicobacter spp. and polypoid growth are related in this study.
In conclusion, an ultrasound of the gastric wall is useful for differentiating hypertrophic pyloric gastropathy from the other pyloric disease. The sonographic and CT features of hypertrophic pyloric gastropathy are intact wall layer and focal wall thickening. Helicobacter spp. in the stomach can induce hypertrophy or chronic inflammation. When thickened muscular layer with intact wall layering of the pylorus was identified on ultrasound and CT in dogs, chronic hypertrophic pyloric gastropathy should be considered in differential diagnosis. Helicobacter test (such as histopathology or kit) is suggested since the bacteria can cause the underlying inflammation. Long-term antibacterial therapy is needed for eradication.













 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



