A retrospective study of 16 cats with intermediate- to high-grade alimentary lymphoma
Article information
Abstract
The purpose of this retrospective study was to describe cases of feline intermediate- to high-grade alimentary lymphoma regarding signalment, clinical presentation, laboratory findings, response to therapy (modified 25-week University of Wisconsin–Madison [UW-25] vs. COP [cyclophosphamide, vincristine, prednisone]), toxicosis, and outcomes and to identify prognostic factors. Sixteen cats were treated with chemotherapy protocols. Response rates and survival did not differ statistically between the two protocols. The progression-free interval (PFI) and median survival time (MST) in cats achieving a response to therapy were longer than in those with no response [NR] (complete remission [CR] vs. partial remission [PR] vs. NR; PFI, 124 vs. 49 vs. 12 days, p < 0.001; MST, 361 vs. 118 vs. 16 days, p < 0.001). Clinical stage was another prognostic factor for PFI and MST. The PFI and MST in cats in stage I were longer than in those in other stages (PFI, 107 days vs. 30 days; MST, 193 days vs. 54 days). Hematologic and gastrointestinal toxicosis was mostly low grade. In comparing the modified UW-25 protocol with the COP protocol, there was not much difference in the number of neutropenic episodes and grade levels.
Introduction
Alimentary lymphoma (AL) is the most common intestinal neoplasm in cats [1-9]. AL comprises a group of diseases present in the gastrointestinal (GI) tract, with mesenteric lymph node and hepatosplenic involvement [1,3]. Although the tumor can be solitary, diffuse forms throughout the intestine are more common [3]. There is no consistent sex bias [3,4,10,11]. Based on the results of histopathology and immunohistopathology, most feline AL can be classified as one of the following three types: 1) low-grade alimentary lymphoma (LGAL), 2) intermediate- or high-grade alimentary lymphoma (I/HAL), and 3) large granular lymphoma. These different subtypes of lymphoma share clinical signs related to GI disorders, commonly as hyporexia, weight loss, vomiting, and diarrhea [1,12-14]. No difference has been reported in clinical symptoms for each subtype; however, I/HAL tends to progress more acutely [1,4,12,15-17].
Treatment protocols for feline AL are variable, but the most common regimens are COP-based (cyclophosphamide, vincristine, prednisolone, or prednisone) or CHOP-based (cyclophosphamide, doxorubicin, vinca alkaloid, prednisolone, or prednisone). However, gold standard chemotherapy protocol does not currently exist [4,6,15,18-26].
The median survival time (MST) differs between the subtypes of lymphoma. The MST of LGAL cats is approximately 1.5 years, whereas that of I/HAL cats treated with multiagent chemotherapy is approximately 4 to 6 months with poor prognosis [4,7,10,15,22-24,27]. In addition, response rates and the durability of response for cats with I/HAL treated with combination protocols are generally not as good as in cats with LGAL. LGAL patients have more than 80% response rates, whereas HGAL patients have a 50% to 65% response rate [10,11,16–18,20-21,23–26,28-30]. In particular, several recent retrospective studies have reported on the response of COP and modified 25-week University of Wisconsin–Madison (UW-25) protocols in cats with I/HAL and demonstrated a good response (response to modified UW-25 and COP protocols: 62% and 92.4%; MST of modified UW-25 and COP, 97 and 112 days, respectively) [11,31].
The following prognostic factors have been reported to be associated with AL: feline leukemia virus (FeLV) antigenemia, grade, treatment response, hypoalbuminemia, and body weight. However, the most consistent prognostic factor is the complete remission (CR) to treatment [10,11,16,17,22,29,30,32].
There is a paucity of studies examining I/HAL. Because the modified UW-25 protocol was developed only recently, to our knowledge, there are no studies that evaluated toxicosis in a modified UW-25 protocol and no research that compared the treatment response between the modified UW-25 and COP protocols in cats with I/HAL. The purpose of this retrospective study was to describe cases of feline I/HAL regarding signalment, clinical presentation, laboratory findings, response to therapy (modified UW-25 vs. COP), toxicosis, and outcomes and to identify prognostic factors.
Materials and Methods
Case selection
Sixteen cats met the inclusion criteria. We reviewed the medical records of cats examined for I/HAL at four institutions, namely, Chungnam National University, Beaksan Feline Medical Center, Korea Animal Medical Center, and Sungsim Animal Medical Center, from 2017 to 2020. Cats with a histologically and cytologically confirmed diagnosis of I/HAL were the subject of this study. Cats with all gross diseases surgically removed were allowed to be included.
Data collection
Patient variables
Collected data were as follows: age, sex, clinical signs at the time of diagnosis, physical examination findings, hematologic data, FeLV, and feline immunodeficiency virus (FIV) testing, thoracic and abdominal radiography, abdominal ultrasound, date of diagnosis, method of diagnosis, clinical stage, date of treatment, response to treatment, date of progression, date of death, cause of death, and adverse events experienced during treatment.
Treatment and outcome
Cats were treated with chemotherapy protocols, including the modified UW-25 or COP protocol. The COP protocol was based on standard doses and intervals [33] and did not exclude patients with a slight modification from chemotherapy (e.g., dose intensity). The modified UW-25 protocol included combination chemotherapy with vincristine, l-asparaginase, cyclophosphamide, doxorubicin, and prednisolone and was based on the report published by Collette et al. [11]. In addition, a slight deviation from the protocol (e.g., the omission of l-asparaginase) did not result in exclusion from this group.
Complete blood count (CBC) was conducted before each treatment. Biochemistry profiles were conducted when the cats showed symptoms associated with toxicosis or other clinical signs.
Exclusion of cats from the response and survival assessment as follows: received no treatment, treated with a chemotherapy protocol other than the modified UW-25 or COP protocol as the first line, their medical records were incomplete.
Response assessment
Cats were objectively evaluated for response to treatment using periodic ultrasonography. Cats were classified based on treatment response as follows: CR, 100% reduction in the size of all measurable disease; partial remission (PR), > 50% but < 100% reduction in the size of all measurable disease; and no response (NR), < 50% reduction in size or an increase in the size of overall measurable disease.
Toxicity assessment
Results of CBC toxicity as well as GI toxicosis were recorded during treatment. Neutropenic episodes were graded according to Veterinary Cooperative Oncology Group-Common Terminology Criteria for Adverse Events (VCOG-CTCAE) v1.1 [34]. Because of the nature of this retrospective study, the grading of GI toxicity according to VCOG-CTCAE v1.1 was not available. Adverse events due to GI toxicity were rated using the following criteria: grade 0, none; grade 1, inappetence, vomiting, and diarrhea that was transient or responsive to dietary management; grade 2, inappetence, vomiting, and diarrhea necessitating medical treatment; grade 3, inappetence, vomiting, and diarrhea necessitating medical treatment and postponement of chemotherapy; and grade 4, inappetence, vomiting, and diarrhea necessitating medical treatment, hospitalization, and postponement of chemotherapy. The attending clinician classified these events as reported in a previous study [21,35].
Statistical analysis
Study endpoints were tumor progression and patient death. The progression-free interval (PFI) was defined as the time between treatment and disease progression. Survival time was defined as the time between treatment and death related to lymphoma. PFI and MST were determined using the Kaplan–Meier product limit analysis. Cats alive at the time of their last follow-up examination or that died for reasons other than lymphoma were censored.
We used univariate Cox forward regression analysis (followed by multivariate Cox forward regression analysis if multiple influential factors were identified) to evaluate the following variables for their independent influence on PFI and MST: age, sex, body weight, clinical stage, FeLV/FIV status, presence of dyspnea, weight loss, lethargy, vomiting at diagnosis, presence of anemia at diagnosis, and CBC and chemistry profiles at diagnosis. We used univariate logistic regression analysis and multivariate analysis when appropriate to analyze the influence of the above factors on whether each cat reached CR. A p-value of < 0.05 was considered significant. All statistical analyses were conducted using IBM SPSS Statistics for Windows, version 25.0 (IBM Corp., USA).
Results
Patient population
Sixteen cats met the requirements for entry into this retrospective study. All cats had either a cytologic or histopathologic diagnosis of I/HAL and were treated with chemotherapy (10 with the modified UW-25 protocol and 6 with the COP protocol). Breeds included Russian Blue (6), Persian (4), Domestic Short Hair (2), Devon Rex (1), American Short Hair (1), Turkish Angora (1), and mixed (1). The median age was 9.5 years (range, 4 to 16 years), and the median body weight was 3.9 kg (range, 2.5 to 6.9 kg). Eleven of the 16 cats were castrated males, and the other five were spayed females. Thirteen of the 16 cats presented with clinical signs of hyporexia, 11 with weight loss, 11 with lethargy, 10 with vomiting, and three with diarrhea. Six cats had FeLV and FIV testing reported. One cat was positive for FeLV. No patient was positive for FIV.
On the day of diagnosis, six of the 16 cats had anemia (hematocrit < 30%). The median hematocrit in all cats was 35% (range, 14.3% to 44.9%). Eight cats were neutrophilic (range, 11.1 to 26.9 K/μL), and five were thrombocytopenic (42 to 126 K/μL).
In the chemistry profiles, azotemia was detected in four cats, elevated liver gamma-glutamyl transferase values were reported in one cat, and hypoalbuminemia was reported in one cat (Table 1).
Diagnosis and clinical staging
Nine cats were diagnosed with I/HAL by biopsy of a lymph node or GI mass, and the other seven cats underwent diagnosis by fine-needle aspiration.
Seven cats had other organ involvement besides the alimentary tract. However, additional tests for staging were not completely performed in some cases. Nine cats had tumors localized to the GI tract. Therefore, all cats were classified into one of the following two categories: stage I and other.
Treatment response
Sixteen cats were available for the evaluation of treatment response and survival analysis. Three cats were censored during the survival analysis. Two patients were still alive and in remission. These cats were in CR at the time of analysis. One cat died of heart failure unrelated to lymphoma while in NR. Two cats were excluded because of denial of treatment and one for incomplete medical records. Rescue therapy was used in five of 16 cats. Three of these cats were treated with lomustine (CCNU), one with L-asparaginase, and one with doxorubicin.
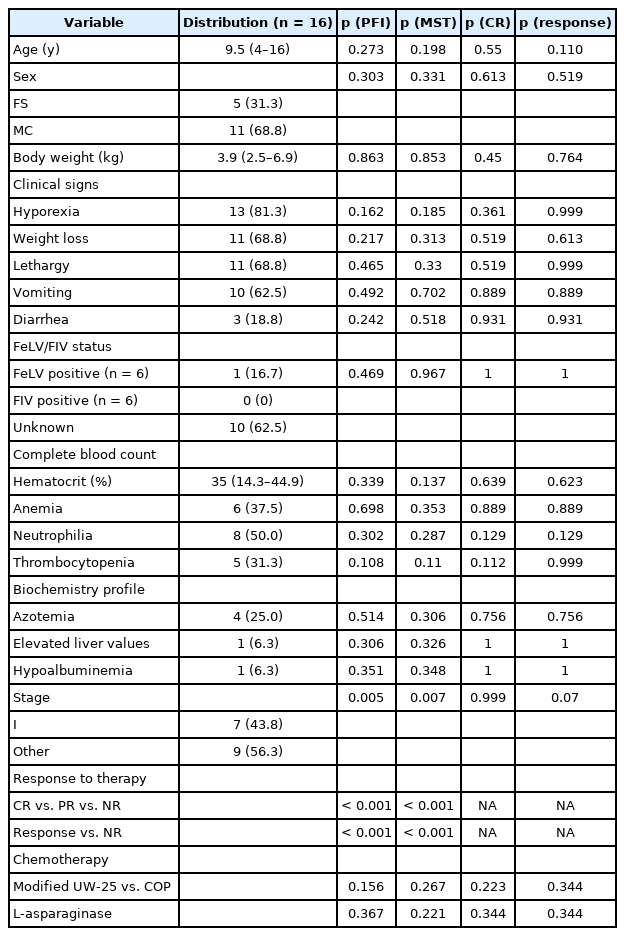
Ten cats received a modified UW-25 protocol, and six cats received a COP protocol. The distribution of the response all 16 patients was as follows: CR, five (31.3%); PR, six (37.5%); and NR, five (31.3%). The overall response rate (CR + PR) was 68.8%. The median PFI (MPFI) was 49 days (range, 9 to 824 days), and the MST was 100 days (range, 9 to 824 days) (Fig. 1).
Kaplan-Meier survival curve of 16 cats with intermediate- or high-grade alimentary lymphoma. (A) Progression-free interval and (B) survival time.
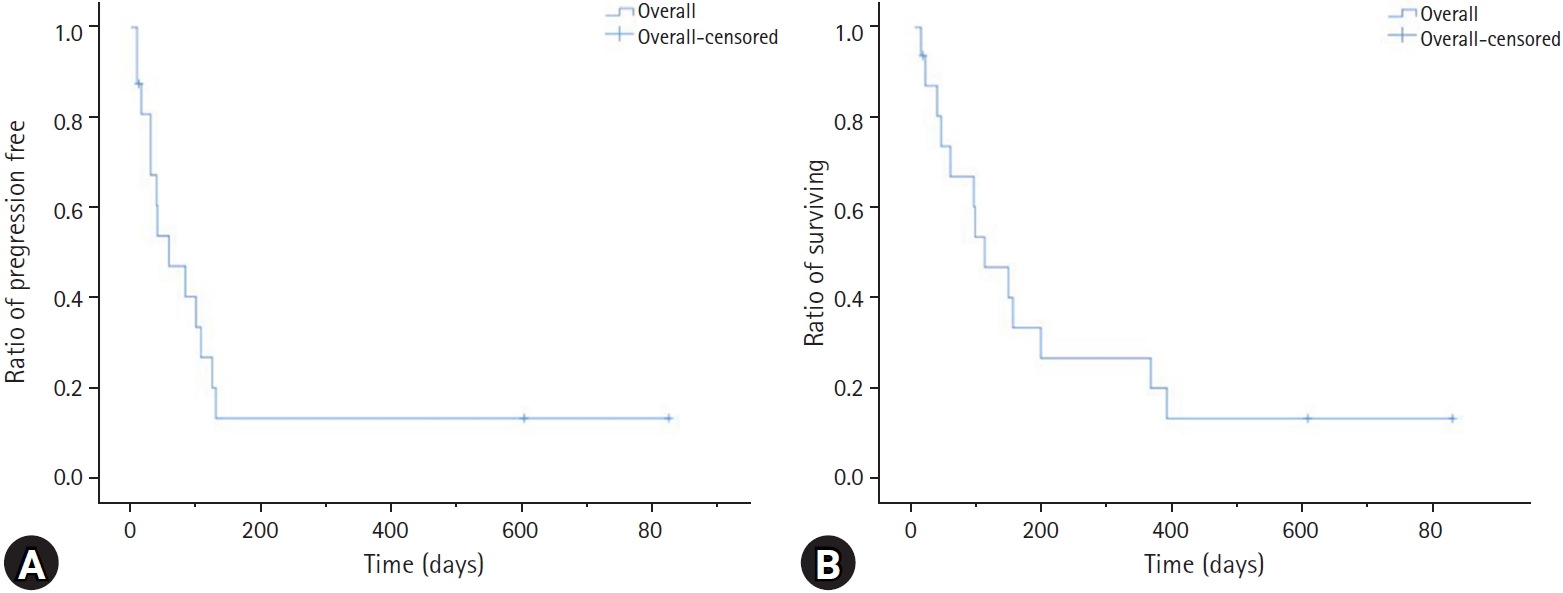
Of the cats treated with a modified UW-25 protocol, two (20.0%) achieved a CR, four (40.0%) achieved a PR, and four (40.0%) did not respond to treatment. The overall response rate (CR + PR) for a modified UW-25 protocol was 60.0%. Among cats receiving a modified UW-25 protocol, the MPFI and MST were 35 days (range, 9 to 824 days) and 65 days (range, 9 to 824 days), respectively. However, of the cats treated with a COP protocol, the response rate was as follows: CR, three (50.0%); PR, two (33.3%); and NR, one (16.7%). The overall response rate (CR + PR) for the COP protocol was 83%. In the cats receiving a COP protocol, the MPFI and MST were 116 days (range, 30 to 602 days) and 172 days (range, 54 to 602 days), respectively (Table 2). There was no statistically significant difference in response rate, PFI, or survival time between the two protocols (Fig. 2).
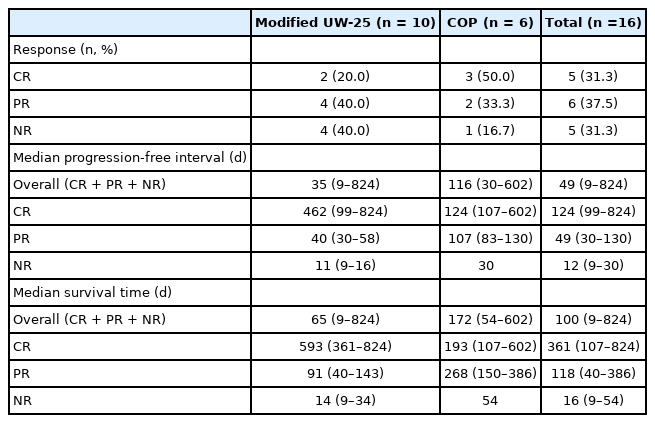
Response rate, remission, and survival duration in cats I/HAL treated with the modified UW-25 or COP protocol
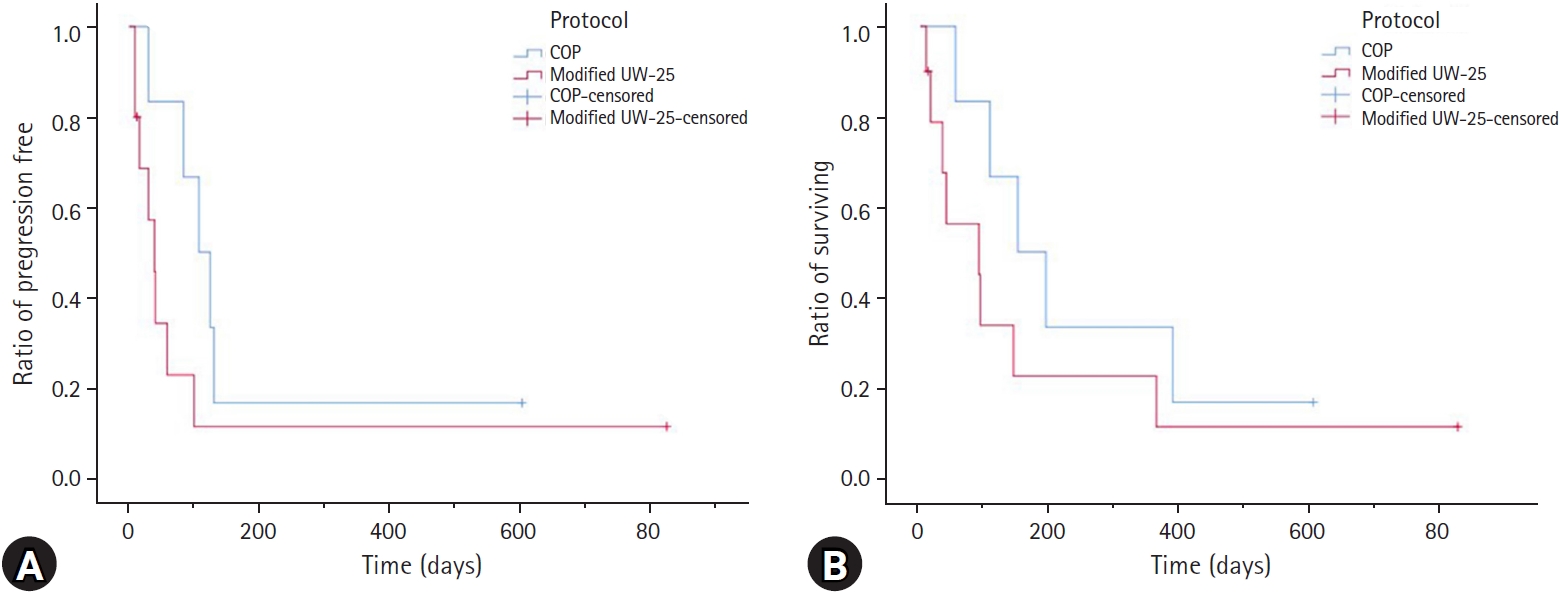
Kaplan–Meier curve of 16 cats with intermediate- or high-grade alimentary lymphoma. (A) Progression-free interval (PFI) and (B) survival time. The blue line indicates cats receiving a cyclophosphamide, vincristine, prednisone (COP) protocol; the red line indicates cats receiving a modified 25-week University of Wisconsin–Madison (UW-25) protocol. There was no significant difference in PFI or survival time between the two protocols (PFI: P = 0.142; median survival time: P = 0.260).
Both the PFI and MST were considerably longer in cats responding to treatment (CR or PR) than in those not responding to treatment (p < 0.001). The MPFI for cats with CR was 124 days (range, 99 to 824 days), in comparison with 49 days (range, 30 to 130 days) for PR and only 12 days (range, 9 to 30 days) for NR. Cats achieving a CR had an MST of 361 days (range, 107 to 824 days), a PR of 118 days (range, 40 to 386 days), and an NR of 16 days (range, 9 to 54 days) (Fig. 3).
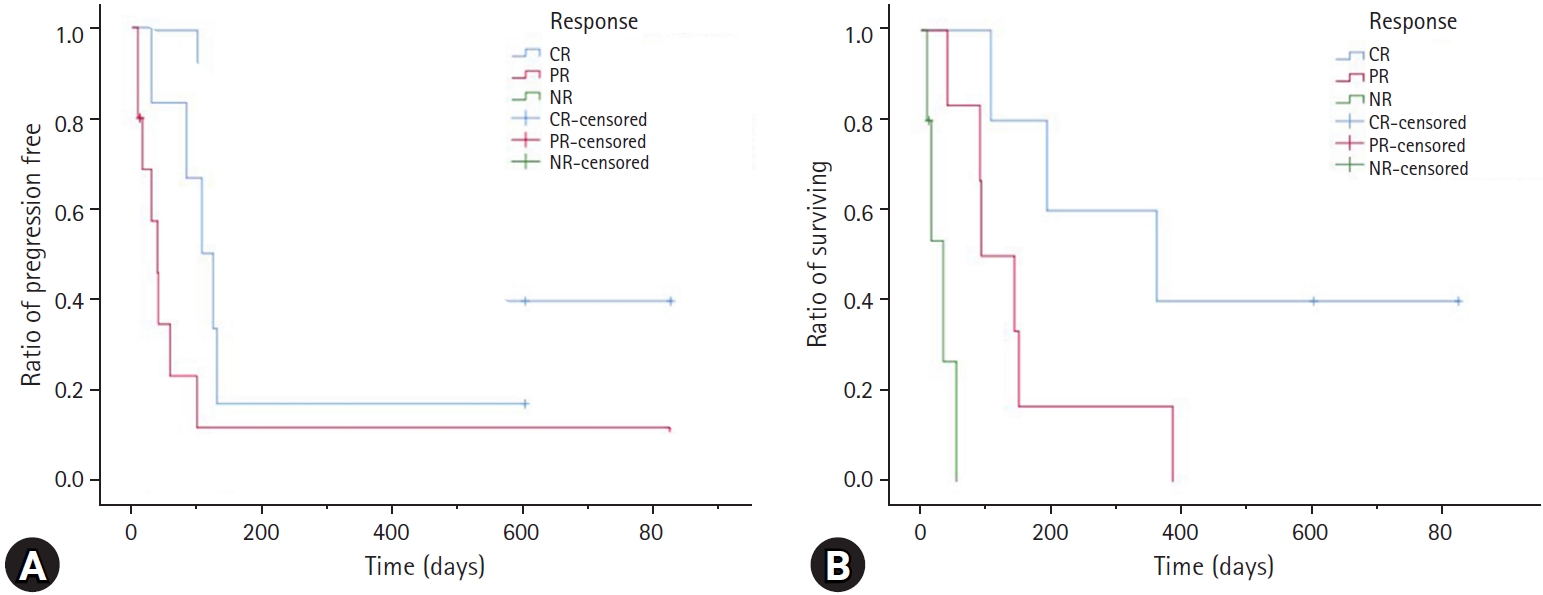
Kaplan–Meier curve of 16 cats with intermediate- or high-grade alimentary lymphoma treated with chemotherapy. (A) Progression-free interval (PFI) and (B) survival time. The blue line indicates cats achieving complete remission (CR), the red line indicates cats achieving partial remission (PR), and the green line indicates cats that had no response to treatment. A significant difference was detected in PFI and survival time between response groups (PFI: P < 0.001; median survival time: P < 0.001).
NR, no response.
Factor analysis indicated that staging was the only factor that affected PFI and MST. The stage I group had a longer PFI and MST than the other stage groups (stage I vs. others; PFI, 107 days vs. 30 days; MST, 193 days vs. 54 days). The other factors were not statistically significant for PFI or MST. No other variables examined were predictive of achieving CR or treatment response (Table 1).
Toxicosis
There were 14 neutropenic events. Nine episodes were documented in 10 patients receiving the modified UW-25 protocol and five episodes in six patients receiving the COP protocol. The median number of episodes per cat was 0.5 (range, 0 to 5). Eight cats (five in the modified UW-25 group and three in the COP group) had no neutropenic episodes. Neutropenia was grade 1 in 71.4% (n = 10), grade 2 in 7.1% (n = 1), grade 3 in 14.3% (n = 2), and grade 4 in 7.1% (n = 1). In groups receiving the modified UW-25 protocol, the number of neutropenic episodes was six in grade 1, one in grade 2, and two in grade 3. In groups receiving the COP protocol, there were four neutropenic episodes in grade 1 and one in grade 4. The distribution of the neutropenic events in cats treated with the modified UW-25 protocol was none after treatment with vincristine and L-asparaginase, seven with vincristine, one with cyclophosphamide, and one with doxorubicin. The distribution of the neutropenic events in cats treating with the COP protocol were three events after treatment with vincristine and cyclophosphamide and two events with vincristine (Table 3).
There were 25 adverse events of GI toxicity documented among all 16 cats (15 in patients receiving the modified UW-25 protocol and 10 in the COP protocol). The median number of events per cat was 1 (range, 0 to 8). Five cats (three in the modified UW-25 group and two in the COP group) had no episodes of GI toxicity. The number of adverse events of GI toxicity was grade 1 in 52.0% (n = 13), grade 2 in 36.0% (n = 9), grade 3 in 8.0% (n = 2), and grade 4 in 4.0% (n = 1). In 10 cats treated with the modified UW-25 protocol, four episodes occurred after induction treatment with vincristine and l-asparaginase, seven with vincristine, three with cyclophosphamide, and one with doxorubicin. In the groups treated with the COP protocol, seven episodes occurred after therapy with cyclophosphamide and vincristine and three after vincristine (Table 3).
Regarding weight change during the treatment and follow-up period, eight cats (50.0%) maintained their weight, four cats (25.0%) experienced a weight reduction of > 10% of their baseline weight, and four cats (25.0%) gained weight of > 10%. Weight change did not influence the remission and survival times in the statistical analysis.
Discussion
The clinical features of these 16 cats with I/HAL were comparable with those described in other reports. The median age of cats with I/HAL was 9.5 years, which is consistent with the recently noted shift in age ranges [17,36]. Hyporexia (81.3%), weight loss (68.8%), lethargy (68.8%), and vomiting (62.5%) were the most common clinical signs reported in the present study. This is similar to previous reports of AL, except for diarrhea (19% vs. 70% to 90%) [1,12-14]. Siamese have a higher risk of developing lymphoma. However, they represent a low percentage of AL. No Siamese cats are included in this study. In recent studies, Siamese cats also represented a low percentage in AL [1,2,7,37]. This may reflect the risk in Siamese cat developing AL is low [14,16,17,36]. Another possibility is that, because of the small sample size in this study, Siamese may not be included in the breed. Although most cases in other papers appear to be Domestic Short Hair, the Russian Blue breed accounted for the largest percentage of cats in this paper [1,2,7,37].
In addition, the cats with I/HAL in this report were mostly males (11/16), and if the same proportion of females and males visited the hospital, the results might suggest that males appeared to be overrepresented. However, data on the proportion of male and female cats that presented to each institution were not available, and because of the small sample size of this study, this result should be interpreted with caution.
Neutrophilia (50.0%), anemia (37.5%), and thrombocytopenia (31.3%) were common findings in the present study. These results were also seen in other reports [1,4,14,15,38].
The overall combined response rate to the modified UW-25 and COP chemotherapy protocols was 69%, and 31% of patients achieved a CR, findings that are similar to the results of other studies [10,11,16,17,22,30]. The CR rate achieved with the modified UW-25 protocol was 20%, which is comparable with that of another study (25%) [11]. The observed CR rate in cats receiving the COP protocol (50%) was higher than what has previously been reported (32%) [4]. The overall PFI and MST in treated cats were 49 and 99 days, respectively. The PFI and MST with the modified UW-25 protocol were 35 and 65 days, respectively, which was shorter than that reported by another study (50 and 85 days, respectively) [11]. The observed PFI and MST in cats receiving the COP protocol (116 and 172 days) were longer than in a previous study (MST, 50 days; PFI was not mentioned) [4]. These data show that the prognosis of cats with intermediate- to high-grade lymphoma is poor. There was no statistically significant difference in CR rate, PFI, or MST between the two protocols. This result raises doubts about the utility of doxorubicin. However, the small number of cases might have reduced the probability of detecting a difference between the two protocols. There was little information associated with both protocols in feline I/HAL. A future investigation that includes a larger group of cats is required to confirm these findings.
We examined only a few prognostic factors in this study. Treatment response is one of the most consistent factors in feline lymphoma studies [3,11,17]. Cats that responded to therapy experienced significantly longer PFI and MST. Therefore, the evaluation of the treatment response is important for predicting prognosis. Clinical stage was also determined to be a prognostic factor for PFI and MST. Cats in stage I had longer PFI and MST than those in other stages (PFI, 107 vs. 30 days; MST, 193 vs. 54 days). Other studies have shown that stage I disease is associated with a more favorable prognosis [6,16,23]. This result lends weight to the importance of the assessment of clinical stage. No other prognostic factors affected PFI or MST. One limitation of the present study is that the low number of cats might have resulted in a lack of power to detect the significance of other prognostic factors.
In this study, hematologic and GI toxicosis were mostly low grade and comparable to that reported in previous studies [21,29]. The median number of neutropenic as well as GI adverse events in each cat was similar to previous reports [21] and as lower than in another report [29]. Different chemotherapy protocols can be a possible consequence. In comparing the modified UW-25 protocol with the COP protocol, there was not much difference in the number of neutropenic episodes and grade levels. Therefore, these two therapeutic regimens were considered to be protocols with acceptable tolerability. In this paper, weight loss before beginning chemotherapy or weight change during treatment or baseline body weight did not affect the outcome. However, one study has shown that a history of pretreatment weight loss has a negative effect on achieving a CR [25]. In addition, studies have reported that baseline body weight or weight change during treatment has an effect on prognosis [28,39]. These different results might be due to the small number of subjects in the present study. The other possibility is that although this paper dealt with only I/HAL, previous papers included lymphoma with various anatomic forms or different grades. A large study is expected to determine the effect of weight on the outcome with I/HAL.
The alimentary form is most common in feline lymphoma, but there are very few papers evaluating the alimentary form alone, especially in I/HAL with multiagent chemotherapy. This is the first study to evaluate the toxicosis of the modified UW-25 protocol. However, there are several limitations of the present study. Limitations of this study include its multi-institutional and retrospective nature. Staging was often limited by the cost and refusal of further examination by the client. Another major limitation is the lack of standardized timing of evaluating the treatment response. A lack of consistency in monitoring is an intrinsic feature of all retrospective studies. Another limitation is that various protocols were applied in the rescue treatment. Five cats (31.3%) received rescue therapies. This unstandardized rescue protocol would likely influence each patient’s survival time differently.
In conclusion, sixteen cats with I/HAL diagnosed by cytologic or histopathologic met the requirements for entry into this retrospective study. I/HAL was found in various breeds, mostly in Russian Blue. Feline I/HAL was distributed in age with 4 to 16 years and had prevalence in old age. I/HAL treated with multiagent chemotherapy had a poor prognosis (MPFI, 49 days; MST, 100 days). There was no statistically significant difference in treatment response and survival between the modified UW-25 and the COP protocol. Hematologic and GI toxicosis during treatment with these two protocols were mostly low grade. There was no significant difference between two protocols. In this study, clinical stage and treatment response were prognostic factors in cats with I/HAL.
Notes
The authors declare no conflict of interest.
Acknowledgements
We are thanks to the “Cooperative Research Program of Center for Companion Animal Research” (Project no. PJ01404502): Rural Development Administration, Republic of Korea. Thanks for sharing the medical charts to “Korea Animal Medical Center”, Cheongju, Korea.