Isolation, characterization and neutralizing activity of porcine epidemic diarrhea viruses from Vietnam
Article information
Abstract
Porcine epidemic diarrhea (PED) is characterized by acute enteritis, watery diarrhea, weight loss, dehydration, and death with high mortality in neonatal piglets. In this study, 3 virus isolates collected in Vietnam between 2016 and 2017 were successfully propagated in Vero cells at high virus titers. Sequence analysis of the full-length spike (S) gene revealed that all 3 isolates belong to genogroup 2a, which is closely related to other prevalent Asian strains. Amino acid sequence comparisons revealed 98.19% to 99.13% homology with the Vietnam isolates circulating during 2013–2015, suggesting that field PED viruses (PEDVs) evolve continuously. Experiments in animals demonstrated that antisera from guinea pigs immunized with the vaccine strain resulted in higher levels (5 log2) of neutralizing antibody against the homologous strain, and showed a relatively lower level of neutralizing antibody against the field isolates. This finding would be helpful in choosing a PEDV strain for vaccine development.
Introduction
Porcine epidemic diarrhea virus (PEDV) causing porcine epidemic diarrhea (PED), is a large-enveloped RNA virus, which belongs to the genus Alphacoronavirus, family Coronaviridae, order Nidovirales. The PEDV genome is approximately 28 kb long containing at least 7 open reading frames (ORF1a, ORF1b, and ORF2–6), and a 3' untranslated region [1,2]. PEDV infection in pigs causes watery diarrhea, anorexia, and depression leading to high mortality rates in suckling piglets [1,3].
It is critical to propagate PEDV isolates efficiently in cell culture to perform research on pathogenesis and vaccine development, as growing PEDV in cell culture has proven difficult. PEDV can be isolated from clinical samples; however, the virus may gradually lose infectivity upon further passages in cell culture [4].
The spike (S) protein of PEDV interacts with the cellular receptor in the entry process of virus and involves in production of neutralizing antibodies in pigs [1]. The S protein is considered a primary target antigen for the induction of neutralizing antibodies and development of an effective vaccine against PEDV [5,6]. Recent reports have demonstrated the diversity of S gene in many isolates. The pathogenicity and antigenicity of PEDV can be changed according to mutations, deletions and/or insertions of amino acids in the S protein. As a result, viral antigenicity and viral neutralizing activity may be changed [7,8]. Zhang et al. [8] in 2015 stated that a field CHN/FL2013 strain reduced virulence in cell culture with a 7-amino acid deletion at N-termination of S protein.
In this study, PEDV isolates from Vietnam were propagated in Vero cells, and full-length S genes were sequenced to investigate the diversity of PEDVs. In addition, the cross-neutralizing activity of the serum of guinea pigs immunized with a PEDV vaccine strain against the isolates was evaluated.
Materials and Methods
Ethics statement
Animal experiments were approved by the Institutional Animal Care and Use Committee (approval number: KW-181031-1) and performed at the Center for Animal Experiment, Kangwon National University.
Isolation of porcine epidemic diarrhea virus in Vero cells
Stool specimens and small intestine were taken from suckling piglets and post-weaning pigs exhibiting acute watery diarrhea. The PEDV HID9047 and HID9048 isolates used in this study were collected from infected samples in Ha Nam and Thai Binh provinces, Vietnam in 2016, and the PEDV HID9049 isolate was collected from infected samples in Hung Yen province, Vietnam in 2017.
Isolation and propagation of PEDV in Vero cells was conducted as adapted from methods in Park et al. [9] in 2018 with modifications. Briefly, 80% confluent Vero cells were inoculated with virus at 37°C for 1 hour. Infection medium (Dulbecco’s modified Eagle’s medium-DMEM supplemented with 0.02% yeast extract, 0.3% tryptose phosphate broth, 5 μg/mL trypsin and 1% antibiotic–antimycotic) was added to the infected cell flask without removing the inoculum. The infected cells were incubated at 37°C for 7 days until extensive cytopathic effects (CPEs) were observed; viruses were harvested by 3 freeze-thaw cycles.
The presence of PEDV was confirmed by a reverse transcription polymerase chain reaction (RT-PCR) amplifying approximately 900 bp of the N gene with primers PEDV-N–F (5′-CGG TTC TCA CAG ATA GTG A-3′) and PED-N-R (5′-CTC CTC CAC TCT GGG ATG T-3′).
Virus titration
Vero cells (1 × 105) were seeded in each well of a 96-well plate (Corning, USA) and grown at 37°C 1 day before the assay. Virus was diluted in infection medium and 0.2 mL of 10-fold serial virus dilutions was added to each well after removing the medium. Each virus dilution was used to infect 10 wells of monolayer Vero cells. CPE was examined under a microscope every day for 7 days postinfection and the virus titer was calculated as the 50% tissue culture infective dose (TCID50) using the Reed-Muench method [7].
Immunofluorescent assay
Vero cells were infected with PEDV 24 hours before the assay. The control and infected cells were fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100. Cells were then blocked with 5% bovine serum albumin, followed by incubation with 1/1,000 monoclonal mouse anti-PEDV (Median Diagnostics, Korea) and 1/1,000 fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse immunoglobulin G (IgG) (MP Biomedicals, USA). The signals were observed using fluorescence microscopy [9].
Nucleotide sequence analysis
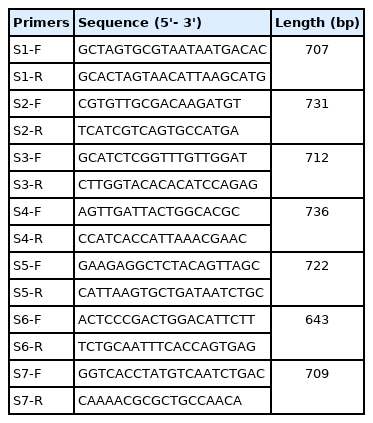
The full-length S genes of PEDVs were amplified by RT-PCR using the primers described in Table 1. The individual fragment was cloned into T&A cloning vector (RBC; Real Biotech Corp., Taiwan), and sequenced in both directions. The sequences of full-length S genes of PEDV isolates in this study and reference PEDVs were used in sequence alignments and phylogenetic analyses with the Serial Cloner 2.6, the ClustalX 2.0 program, and the Mega 6.0 program [10].
Serum neutralization
The neutralizing activity of antisera collected from guinea pigs immunized with a PEDV vaccine strain SM98 against PEDV isolates HID9047, HID9048, and HID9049 was evaluated. Briefly, 3 to 4 month-old guinea pigs (n = 3) were immunized twice intramuscularly at a 2-week interval with 105 TCID50 inactivated vaccine strain SM98. Two other guinea pigs were given phosphate-buffered saline (PBS) as control. Serum samples were collected 2 weeks after the last immunization for the neutralizing assay.
Vero cells were grown at 2 × 105 cells per well in 96-well plates. Viruses were diluted to 200 TCID50 in 50 µL DMEM, mixed with 50 µL of twofold diluted serum and incubated at 37°C for 1 hour. The cells were rinsed 3 times with PBS, followed by inoculation of 100 µL of the mixture at 37°C for 1 hour. After removing the mixture, the cells were rinsed 3 times with PBS, and the infection medium was added to the cells and incubated at 37°C, 5% CO2. The neutralization titer was calculated as the highest dilution of serum that inhibited virus-specific CPE [10].
Statistical analysis
One-way analysis of variance in GraphPad Prism 6 (GraphPad Software Inc., USA) was used, and p-values of less than 0.05 were considered statistically significant.
Results
Virus isolation and characterization
PEDV-PCR positive samples were processed and incubated in Vero cells. Three PEDV isolates, designated HID9047, HID9048, and HID9049, were propagated successfully in Vero cells with clear CPEs such as cell fusion, large syncytia and cell detachment (Fig. 1A). The viral titers of HID9047, HID9048, and HID9049 were 107.5, 108.2, and 108.5 TCID50/mL at passage 10, respectively. To confirm virus growth in Vero cells, an immunofluorescence assay was performed using the monoclonal mouse anti-PEDV and FITC-conjugated goat anti-mouse IgG antibodies. A specific fluorescence signal was detected in the cytoplasm of Vero cells infected with viruses, but not in the non-infected cells (Fig. 1B).
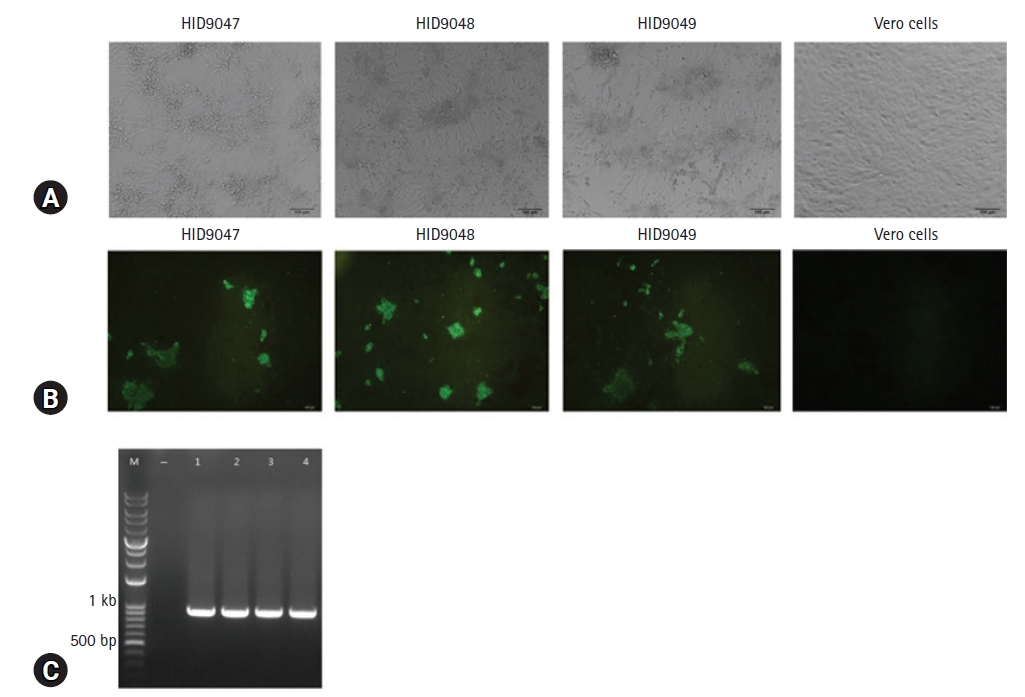
(A) Isolation of Vietnam porcine epidemic diarrhea virus (PEDV) isolates in Vero cells at 5 days postinfection with obvious cytopathic effects such as cell fusion and large syncytia. (B) Immunofluorescence assay in Vero cells using monoclonal mouse anti-PEDV and fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin G antibodies. (C) Reverse transcription polymerase chain reaction amplifying a 900 bp fragment of the N gene. M, DNA standard 3 kb plus; , Vero cell control; 1, SM98; 2, HID9047; 3, HID9048; 4, HID9049. Scale bar: (A, B) 100 μm.
Phylogenetic analysis
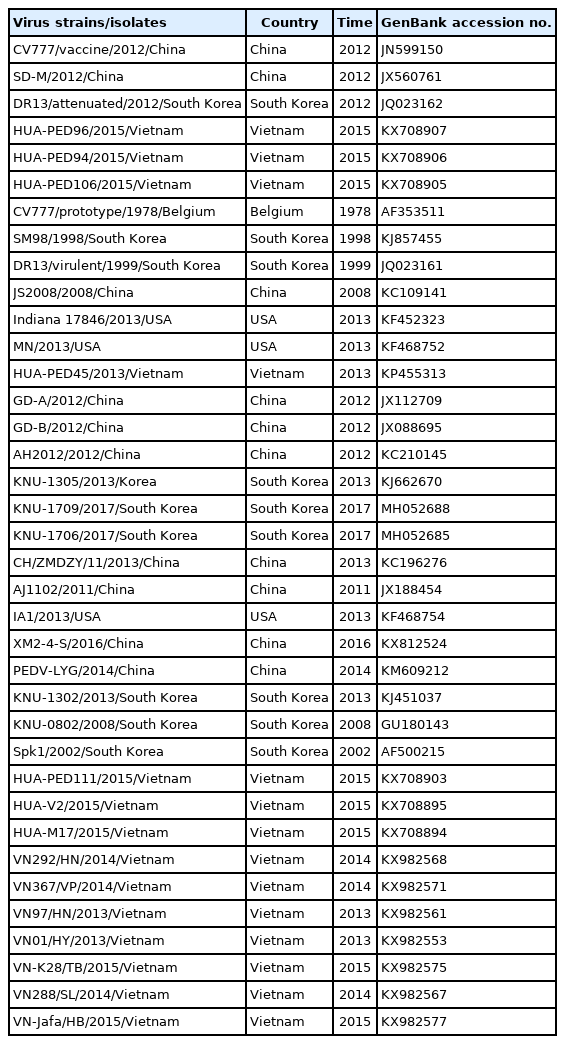
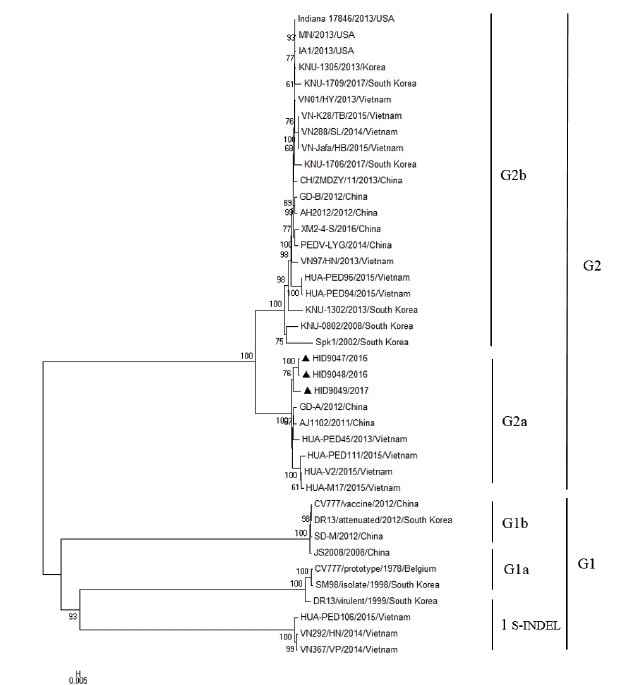
A total of 40 PEDVs were used for the construction of a phylogenetic tree (Table 2). In this study, phylogenetic analysis based on the nucleotide sequences of the S gene revealed that PEDV strains could be divided into 2 genogroups, the classical genogroup 1 represented by prototype CV777 and the variant genogroup 2 of pandemic strains. Each group can be divided into subgroups 1a, 1b, 1 S-INDEL, 2a and 2b. Three isolates (HID9047, HID9048, and HID9049) were found to belong to the subgroup 2a, which is most closely related to the Asian strains designated HUA-PED45/2013/Vietnam, HUA-PED111/2014/Vietnam; GD-A/2012/China and AJ1102/2011/China (Fig. 2).
Spike gene sequence comparisons
The entire DNA sequences of the S genes and the deduced amino acid sequences of 3 isolates from Vietnam were compared with the sequences of the reference strains (CV777, SM98, DR13) and other Vietnam isolates. As shown in Table 3, the nucleotide identities of the S gene between 3 Vietnam isolates and reference strains ranged from 91.80% to 92.71%, and the deduced amino acid sequences were 96.74% to 97.61% homologous. Furthermore, compared with the Vietnam isolates (HUA-PED45, HUA-PED111, VN97, and VN-K28), the 3 isolates shared 96.78% to 97.52% nucleotide homology and 98.19% to 99.13% amino acid homology. The homology of nucleotide and amino acid of 3 isolates showed 98.15% to 99.76% and 99.06% to 99.64%, respectively (Table 3).
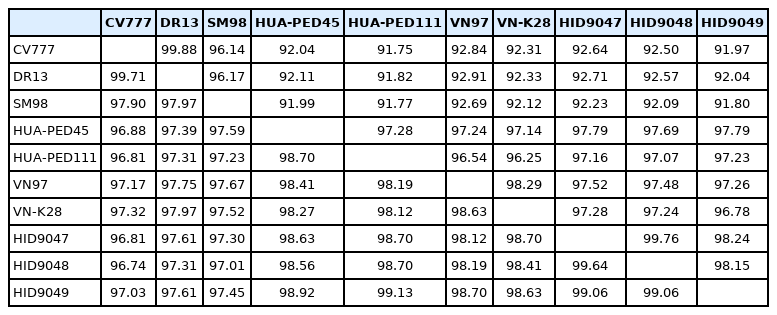
Pairwise comparisons of the nucleotide and deduced amino acid sequences of the full-length S gene of porcine epidemic diarrhea virus isolates compared with the reference strains
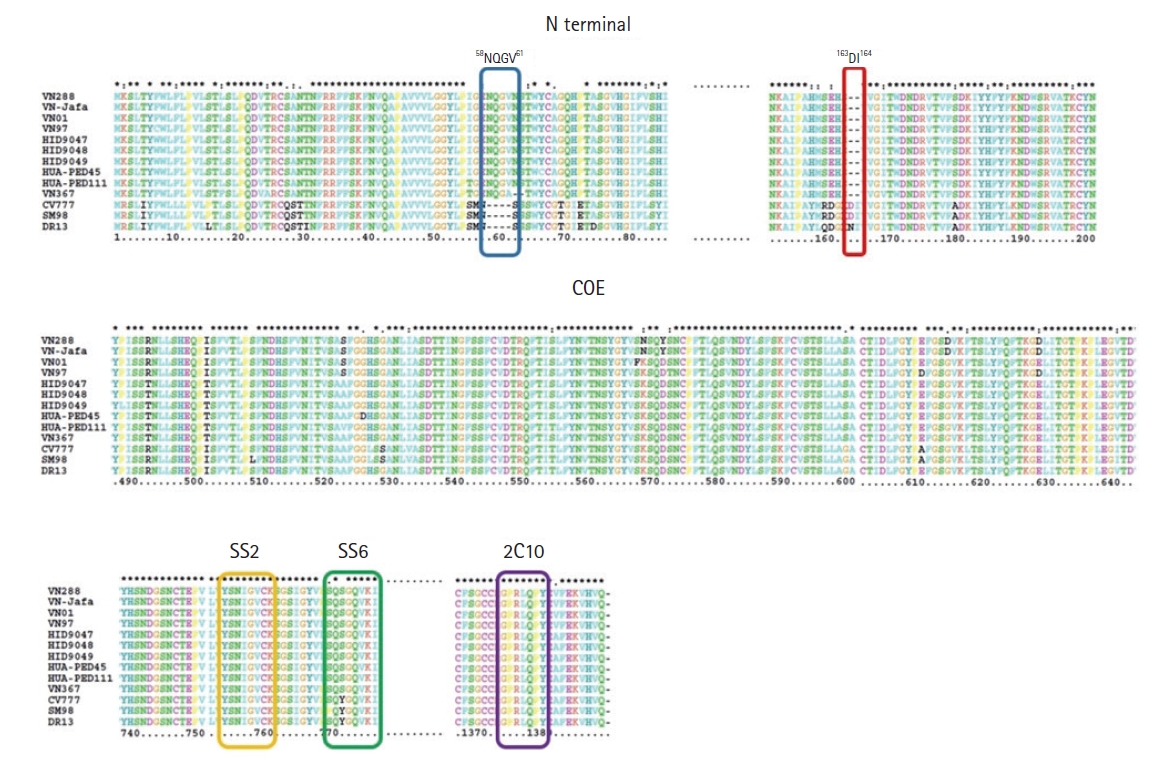
Sequence alignment of amino acids in the S protein illustrated that mutations in amino acid sequence including substitutions, insertions and deletions were observed in 3 Vietnam isolates. Among them, 4 insertions (58NQGV61) and 2 deletions (163DI164) were found in the N-terminal of S protein (Fig. 3). Alignment analysis of 4 neutralizing epitope regions COE, SS2, SS6 and 2C10 revealed that all 3 Vietnam isolates in this study have 4 substitutions in COE (502I → 502T, 509L → 509S, 529S → 529G, 607A → 607E), 1 substitution in SS6 (766Y → 766S), and no mutations in SS2 and 2C10 compared with CV777, DR13, and SM98 strains (Fig. 3). Comparison with other Vietnam strains (VN288, VN-Jafa, VN01, VN97 isolated during 2012-2015) indicated the substitution in COE (502I → 502T), and the conservation in SS2, SS6, and 2C10 region. PEDV VN288, VN-Jafa, VN01, VN97 showed addition substitutions in COE, while HUA-PED45 showed more homology with the 3 isolates, suggesting that 3 isolates HID9047, HID9048, and HID9049 are closely related to PEDV HUA-PED45.
Neutralizing activity
To determine whether vaccine strain SM98 can neutralize the 3 PEDV isolates (HID9047, HID9048, and HID9049), guinea pig antisera collected 2 weeks after the final immunization were used for neutralizing tests. The antisera effectively inhibited infection with the vaccine strain SM98 with mean neutralizing antibody (NA) titers of 5 log2. The antisera showed lower dilutions with mean of NA titers of 3.67 log2 (p < 0.01), 4 log2 (p < 0.05), and 3.27 log2 (p < 0.001) for inhibiting infection with isolates HID9047, HID9048, and HID9049, respectively (Fig. 4). There was a significant difference in the mean NA titers against the SM98 strain and 3 isolates, suggesting that the antisera strongly reacted with the homologous virus, but moderately with the heterologous field isolates regarding to antigenic variations between PEDVs.
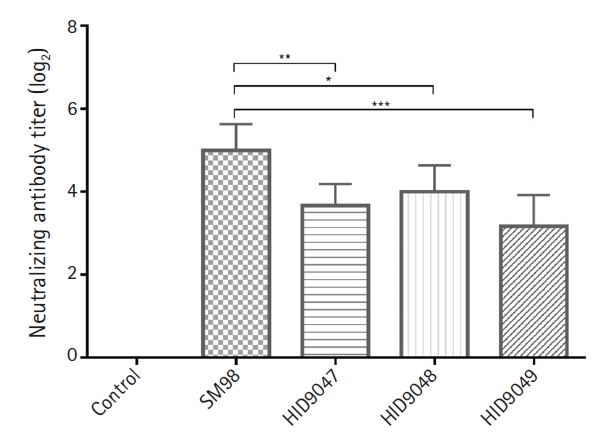
Neutralizing antibodies in serum samples of guinea pigs immunized against porcine epidemic diarrhea virus (PEDV) isolates. Serum samples were collected at 2 weeks after the second immunization and subjected to the virus neutralization assay using isolates PEDV HID9047, HID9048, and HID9049. Data represent the mean standard deviation. Statistical significance was assessed by one-way analysis of variance. *p < 0.05, **p < 0.01, ***p < 0.001.
Discussion
The S protein is an essential factor mediating PEDV entry into host cells and is considered a primary target antigen for induction of neutralizing antibodies and the development of an effective vaccine against PEDV [5-7]. Several previous studies indicated that the S gene is prone to mutations including replacement, insertion and/or deletion of nucleotide sequences [7,8,11,12].
PEDV has been reported in Vietnam since 2010 and has spread rapidly [13]. The disease has been circulating and becoming a significant issue in the pork industry. This study was conducted to isolate PEDVs from infected pigs in Vietnam, investigate the genetic variation of PEDV isolates based on the full-length S gene and assess the neutralizing activity of the traditional vaccine strain with the circulating isolates.
In this study, 3 PEDV isolates were successfully isolated from infected samples and propagated in Vero cells with high virus titers. Nucleotide sequencing and a phylogenetic tree based on the S gene revealed that the 3 isolates from Vietnam (HID9047, HID9048, and HID9049) belong to subgroup 2a, which includes PEDV isolates that circulated in China from 2011 to 2012 and in Vietnam from 2013 to 2015. In a previous study conducted by Diep et al. [13] in 2018, highlighting the genetic heterogeneity of PEDVs in Northern Vietnam from 2012–2015, 6 out of 25 isolates were found to belong to subgroup 2a, which is closely related to Asian strains. Our 3 PEDVs were isolated in 2016 and 2017, suggesting that they are novel PEDVs that share unique genetic features with the subgroup 2a PEDV strains circulating in Vietnam from 2013 to 2015. In addition, the 3 isolates showed less mutations than PEDV HUA-PED111, HUA-V2, and HUA-M17 which were isolated from in the northern provinces of Vietnam in 2015, while showed higher homology in amino acid sequences with strain HUA-PED45, suggesting that the 3 isolates and PEDV HUA-PED45 are genetically closely related. Amino acid sequences alignment indicated mutations such as substitutions, mutations, insertions and deletions in S protein, especially in N-terminal region. N-terminal domain interacts with 5-N-acetylneuraminic acid, mutations in N-terminal may have impact on PEDV infection in the host [14].
Neutralizing activity of antisera against the 3 Vietnam isolates was determined. Immunized sera showed the higher neutralizing activity against the homogenous strain SM98, but moderate against the isolates HID9047, HID9048, and HID9049. This finding is consistent with a previous study that revealed that the antisera strongly recognized the homologous strain regarding to antigenic variations [10]. In a study of Lee et al. [10] in 2015, antisera of guinea pigs given 2 immunization with strain KNU-141112 effectively inhibited the homogenous strain with mean NA titers of 1:112, but relatively low NA titers of 1:37 against strain SM98-1. In this study, the 3 isolates have 1 and 4 amino acid substitutions in SS6 and COE regions, respectively, which may support the hypothesis of variations in neutralizing epitope and speculate that the changes may influence the antigenicity of PEDV. Further studies on pathogenicity and neutralizing activity of these 3 isolates need to be carried out in pigs.
Notes
The authors declare no conflict of interest.
Acknowledgements
We would like to acknowledge the veterinarians who collected the infected samples and the contribution of all authors for this study.