Vegetative endocarditis associated with Arcanobacterium haemolyticum in a Holstein cow
Article information
Abstract
A 4-year-old Holstein cow with progressive atrophy and ataxia was submitted for diagnosis. The postmortem examination revealed multifocal yellowish nodules in the pulmonary parenchyma and vegetative masses in the mitral and tricuspid valve of the heart. Both kidneys were severely enlarged, with multiple yellow nodules on the parenchyma. Histopathologically, pulmonary abscesses, vegetative endocarditis, suppurative glomerulonephritis, and fibrino-purulent arthritis were observed. The tiny β-hemolytic bacterial colonies were isolated from the lesions and identified as Arcanobacterium haemolyticum by the VITEK 2 system (bioMérieux, USA). This is the first documented report of an A. haemolyticum infection in a Holstein cow in Korea.
Arcanobacterium haemolyticum, formerly known as Corynebacterium haemolyticum, is a β-hemolytic Gram-positive, catalase-negative, facultative-anaerobic, non-acid-fast bacilli. This bacterium can cause a wide range of diseases in humans and has been implicated as a cause of wound infections but rarely of systemic infections, including endocarditis, meningitis, osteomyelitis, and pneumonia [1,2]. On the other hand, A. haemolyticum infections have rarely been reported in animals, and the pathogenicity of this bacterium in animals has not been documented. Reports of sporadic isolations from animals, including cattle [3], sheep [4], a pet rabbit [5], horses [6,7], and a badger [8], have been published. In Korea, several cases of A. haemolyticum infections have been reported in humans, including a case of sepsis [9], 5 cases of skin ulcer and peritonsillar abscesses [10], and a case of necrotizing fasciitis in a dog-bitten patient [11]. This paper describes a case of vegetative endocarditis associated with an A. haemolyticum infection in a Holstein cow in Korea.
A 4-year-old female Holstein cow with a 1-month history of progressive atrophy, lethargy, depression, and ataxia was submitted for diagnosis. At necropsy, the cow showed a poor body condition, the considerable depletion of subcutaneous adipose tissue, and adjacent dark reddish-brown muscles. In the lungs, there were multiple well-circumscribed yellowish abscesses approximately 5 mm in diameter. The heart was moderately enlarged with a round apex. Severe irregular protruding multiple rough cauliflower-like yellowish–red nodules, approximately 3 × 2.5 × 1.5 cm in size, were attached to the mitral valve of the left heart (Fig. 1A). Similar nodules, approximately 4 × 4.5 × 2 cm in size, were observed on the tricuspid valve of the right side of the heart (Fig. 1B). Both kidneys were severely enlarged and had multiple small raised white-yellow nodules as large as 0.3 cm in diameter in the parenchyma (Fig. 1C). The acetabular fossa of the right hind limb contained large amounts of turbid yellowish fluid and fibrinous materials (Fig. 1D). No remarkable changes were noted in the other organs, including the urinary bladder and liver.
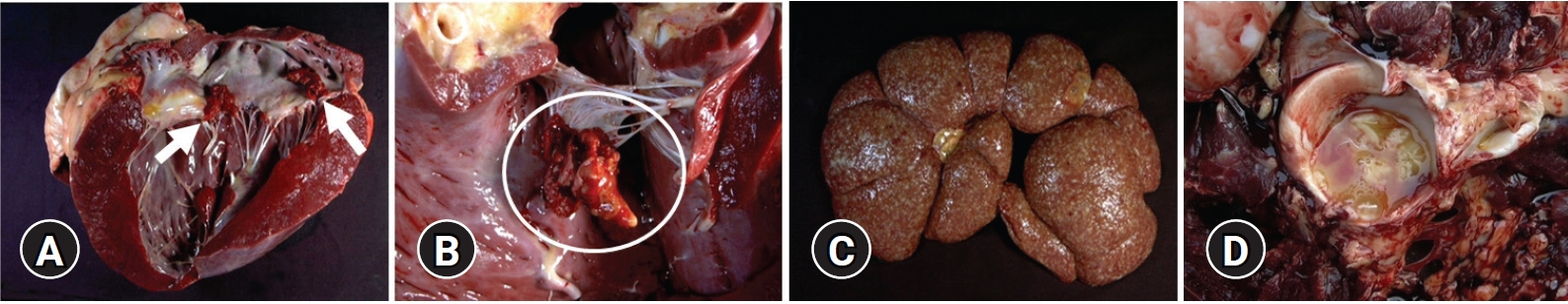
Gross findings. (A) Heart: irregular, raised, rough, yellow-red, friable vegetative masses (3 × 2.5 × 1.5 cm3, arrows) in the mitral valve. (B) Heart: vegetative mass (4 × 4.5 × 2 cm3, circle) in the tricuspid valve. (C) Kidney: multifocal variable-sized yellowish–white nodules up to 0.3 cm in diameter in renal parenchyma. (D) Hip joint: a large amount of turbid yellowish fluid in the acetabular fossa.
For a histopathology examination, the representative tissue samples were fixed in 10% neutral buffered formalin, trimmed, processed routinely, embedded in paraffin wax, sectioned at 3 μm, and stained with hematoxylin and eosin and a Gram staining kit (Brown and Brenn modified method; Sigma Aldrich, Germany). Aseptically collected samples from the lung, vegetative lesions of heart, kidney, and articular swab were cultured on 5% sheep blood agar and MacConkey agar for 48 hours at 37℃ incubation under anaerobic conditions.
Histopathologically, the affected valve leaflets of the heart had lost the endothelial surface and were thickened with granulation tissues composed of fibroblasts, collagen fibers, and some new-formed capillaries. The upper vegetation of the valve was composed of massive fibrin, degenerated neutrophils, and macrophages. Numerous short bacilli-like basophilic bacterial colonies were observed in the lesions of vegetative endocarditis (Fig. 2A) and confirmed as Gram-positive coccobacilli (Fig. 2B) using a Gram stain. Severe multifocal abscesses, suppurative glomerulitis, and tubulonephritis, with bacterial colonies, were observed in the kidney (Fig. 2C). Many renal tubules were filled with proteinaceous fluid, degenerated epithelial cells, and numerous bacterial colonies. Bacterial colonies in the kidney were also confirmed as Gram-positive coccobacilli (Fig. 2C, inset). Multiple abscesses were observed in the parenchyma, including intrabronchial and intravascular areas of the lungs (Fig. 2D). Fibrinopurulent arthritis and multifocal cerebral microabscesses with moderate choroiditis were also presented.
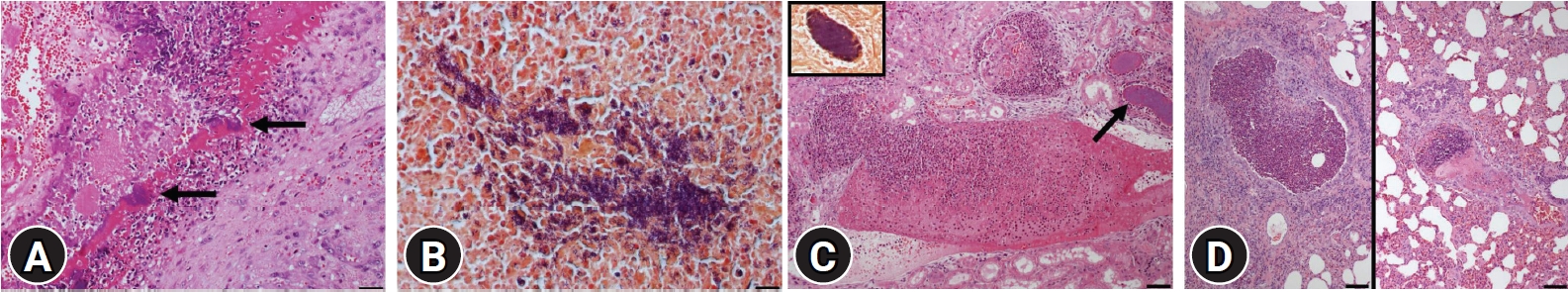
Histopathology findings. (A) Valve of the heart: numerous inflammatory cells, bacterial colonies (arrows), and fibrin deposition in the lesion of vegetative endocarditis (H&E, scale bar: 50 ㎛). (B) Valve of heart: Gram-positive bacilli within the lesion (Gram stain, scale bar: 50 ㎛). (C) Kidney: suppurative inflammation in the glomeruli and bacterial colony (arrow) in the renal tubules (H&E, scale bar: 100 ㎛). Note Gram-positive bacilli in the renal tubule (inset) (Gram stain). (D) Lungs: abscess formation in the bronchiole (left) and blood vessel (right) (H&E, scale bar: 100 ㎛).
In the bacterial culture test, small translucent colonies were observed after 48 hours in anaerobic incubation of the samples from the lungs, valves, kidneys, and articular swabs. The colonies were 0.5 mm in diameter, and a narrow zone of β-hemolysis was observed on all sheep blood agar. The isolated organism was catalase-negative and confirmed as A. haemolyticum (99% probability) by biochemical tests using the VITEK 2 system (bioMérieux, USA).
Based on the gross findings, histopathologic features, and isolation of the bacterial agent, this case was diagnosed as a systemic infection with vegetative endocarditis by A. haemolyticum in a Holstein cow.
A. haemolyticum presents as a commensal on the skin and mucous membrane of the upper respiratory tract in humans. According to the previous literature based on a Medline search, human infections associated with A. haemolyticum were classified into 3 major categories: local infection, systemic bacteremia, and deep-seated infections without bacteremia [1]. Deep-seated infections by A. haemolyticum are usually monomicrobial, whereas soft tissue infections are polymicrobial, associated with other bacteria [12]. Human patients with bacteremia could be classified into 2 groups: an older population with underlying immune-suppressive conditions (usually a malignancy) and a younger population with predominantly upper respiratory tract infections [1,2].
Skin infections have been described mainly after a traumatic skin injury or postoperative wound infection [13]. A. haemolyticum has occasionally been isolated in patients with sepsis, osteomyelitis, septic arthritis, wound infections, skin abscesses, peritonsillar abscesses, pulmonary abscess, pyothorax, endocarditis, brain abscesses, and diabetic soft tissue infections [1,13]. These conditions occur mainly in patients with underlying predisposing diseases, such as diabetes, alcoholism, or malignant neoplasms in humans [13]. According to a retrospective review of the microbiological culture results, 96% of isolates originated from patients with wound infections and/or cellulitis, and 72% of patients had coexisting diabetes mellitus [2].
In animals, A. haemolyticum has been isolated in bovine semen and a case of ovine pneumonia [3,4]. In horses, A. haemolyticum strains have been isolated with other microorganisms from wound infections, dermatitis, a fistula after castration [6], and postcastrational complication [7]. In one study, 12 rabbit mandibular and maxillary abscesses were cultured aerobically and anaerobically. Afterward, A. haemolyticum, a type of periodontal bacteria, was isolated from one specimen [5]. In the present case, A. haemolyticum was isolated from several tissues, such as the heart, lungs, kidneys, and joints. In particular, the histopathological findings, such as endocarditis and glomerulonephritis by this bacterium, have not been reported in animals. Published data on this infection in animals is lacking. Therefore, the mode of transmission and spectrum of the diseases and infections are uncertain.
According to the colony morphology and β-hemolysis on blood agar, A. haemolyticum isolates are divided into 2 different biotypes, such as smooth and rough colony types [14]. The smooth biotypes are β-glucuronidase negative, often ferments sucrose, possess smooth edges, are moderate to strong in β-hemolysis, and are associated with wound infections. In contrast, rough isolates are β-glucuronidase positive, negative for sucrose, possess a rough and irregular edge, have weak to no β-hemolysis, and are associated with pharyngitis [14]. On horse or sheep blood agar, colonies of A. haemolyticum are circular, discoid, opaque, and whitish with a rough surface and friable consistency and averages of 0.1 mm with little or no hemolysis after 24 hours incubation. At 48 to 72 hours, the colonies reach 0.5 mm with a narrow 1 mm zone of β-hemolysis. On the other hand, prominent hemolysis is seen on 5% of human blood agar within 24 hours, and sucrose fermentation is variable [15]. In this case, the A. haemolyticum isolate showed small (0.5 mm diameter) colonies with a narrow zone of β-hemolysis after 48 hours of incubation on sheep blood agar. This bacterium showed a negative result for sucrose on the VITEK test.
A. haemolyticum must be differentiated from Arcanobacterium pyogenes, which is also catalase-negative and may appear β-hemolytic on sheep blood agar. A. pyogenes is a common inhabitant of the mucous membranes of cattle, sheep, swine, and other economically important animals and can disseminate to cause a wide variety of suppurative infections in the skin, joints, and visceral organs. On the other hand, the 2 organisms can be differentiated easily as A. pyogenes hydrolyses gelatin rapidly, produces acid from xylose, and produces β-glucuronidase [14,15].
Endocarditis is defined as an infection of 1 or more in the endocardial surfaces of the heart [16]. In cattle, different predilection sites for valvular vegetation have been reported. A previous paper reported that endocarditis occurs most commonly on the tricuspid (right atrioventricular) valve followed by the mitral (left atrioventricular) valve. Moreover, bilateral involvement of the atrioventricular valves is not common [17]. According to a survey of 36 cows with vegetative endocarditis, the pulmonic, tricuspid, and mitral valves were affected as a single infection in 20 (55%), 9 (25%), and 4 (11%) cases, respectively [16]. In addition, 2 cows (6%) had vegetation at both the tricuspid and mitral valves. In the present study, the lesions of vegetative endocarditis caused by A. haemolyticum were observed in the tricuspid and mitral valves. In cattle, the valves on the right side of the heart are more commonly involved, possibly because of the introduction of bacteria by veterinarians during jugular venipuncture [18]. On the other hand, there is no information on intravenous injection by farmers and veterinarians in the present case. The most common clinical signs in cattle with vegetative endocarditis are weight loss, recurrent fever, anorexia, dyspnea, lameness, and poor milk production [16].
In the previous study, various additional pathological findings were detected in cattle with vegetative endocarditis. Embolic nephritis and pulmonary thromboembolism were observed in 10 (28%) and 9 (25%) cases, respectively. Six cows (17%) had joint effusion, and 5 (14%) had ascites [16]. In the present report, intrabronchiolar and intravascular abscesses were observed in the lungs. In addition, the renal tubules and glomeruli contained numerous bacterial colonies with suppurative inflammation. No typical lesions were detected in the urinary bladder.
Although the source of infection and the precise pathogenesis are not completely understood in this case, the infected A. haemolyticum may invade the blood vessels and cause longstanding irritation to the endocardium resulting in vegetative endocarditis in cattle. The bacterial emboli from endocardial vegetation spread to other internal organs via the bloodstream and then formed multiple abscesses in the lungs, suppurative inflammation in the glomeruli, and arthritis. Several histopathologic features, such as an intravascular abscess in the lungs and suppurative inflammation in the glomeruli, supported the hematogenous spread of A. haemolyticum in this cow.
Although A. haemolyticum can cause infective endocarditis, only 5 cases (3 males and 2 females) have been reported in the medical literature [1,19]. Four out of the 5 cases were complicated by septic embolism. Two men died after developing infective endocarditis with/without cerebral emboli. One man and 2 females survived with prompt antibiotics therapy and surgery. The causes of death in endocarditis caused by A. haemolyticum in humans were cardiac arrest resulting from the progression of congestive heart failure and fatal neurological complications associated with the septic embolism [20]. Recently, a case of subacute infective endocarditis associated with A. haemolyticum on the mitral valve prolapsed and complicated with systemic emboli was reported in a woman in Korea [20]. The most prevalent complication of infective endocarditis in humans is septic emboli to the brain.
Although endocarditis by an A. haemolyticum infection has been reported in humans, it has not been reported in animals. Moreover, the pathogenesis of this organism in animals is unclear. This paper describes an interesting case of a systemic infection of A. haemolyticum with endocarditis in a Holstein cow.
Notes
The authors declare no conflict of interest.
Acknowledgements
This research was supported by the 2021 scientific promotion program funded by Jeju National University.
