Cutaneous metastasis of mammary gland tumor in a dog: a case report
Article information
Abstract
An 8-year-old spayed female, mixed dog presented multiple, bloody exudative skin lesions on the bilateral flank which spread 1 week after mastectomy for treatment of mammary gland tumor (MGT). Multiple, ill-marginated, irregular, and heterogeneously thickened cutaneous and subcutaneous lesions and enlarged lymph nodes were identified in ultrasound and computed tomography. Histopathological examination confirmed adenocarcinoma with lymphatic invasion presumed to be metastatic MGT. Clinical signs improved after chemotherapy but died after 1 month. This study suggests that cutaneous metastasis be considered for differential diagnosis of cutaneous lesions in dogs with a history of MGT, although skin metastasis from MGT is rare.
Canine mammary gland tumor (MGT) is the most common neoplasia, accounting for 50% to 70% of all tumors in intact female dogs [1]. Most malignant tumors are comprised of epithelial tumors or carcinomas [2]. Malignant MGT rarely occurs in dogs aged younger than 5 years of age, and the mean age of dogs with MGT is from 9 years to 11 years [1]. Dogs may present with nonspecific signs, and those with inflammatory carcinoma present with extensive inflammation of the involved mammary glands with edema and pain [3]. In general, MGT metastasizes to the regional lymph nodes and lungs and more distant metastatic sites, such as the liver, spleen, bone, kidney, and brain; however, skin metastasis is rare [1,4]. To date, only 4 reported cases of skin metastasis have been reported in dogs with MGT [4-6].
Although MGT is easily detected through physical examinations, radiography, ultrasonography, computed tomography (CT), fine-needle aspiration of the regional lymph nodes, and biopsy could be performed to evaluate metastasis [1,3]. The ability to detect pulmonary metastasis is improved due to the use of CT which contributes to the diagnosis of early-stage disease [3]. Most MGT metastasis occurs within 1 year of the initial surgery [3]. Surgical removal of the affected tissues remains the most accepted treatment for MGT [1]. Surgical excision alone yields unsatisfactory results in patients with lymphatic invasion due to high recurrence [7]. Several prognostic factors, including age, tumor size and stage, clinical signs, lymph node involvement, histopathologic type, and the presence of hormone receptors, have been identified [4,7]. The present study described the clinical manifestation, diagnostic imaging findings, treatment, and prognosis in a dog with cutaneous metastasis of MGT.
An 8-year-old spayed female, mixed dog was referred to the Veterinary Medical Teaching Hospital of Kyungpook National University for skin lesions. The patient was diagnosed with MGT at a local veterinary clinic and underwent mastectomy and ovariohysterectomy 6 weeks later. Palpable skin lesions on the bilateral flank were detected at the time of surgery and spread of multiple skin lesions presented on the bilateral flank 1 week after operation. The patient’s lesions did not improve even after 1 month of anti-inflammatory treatment for the skin lesions. Painful, crust, bloody exudate, and multifocal nodules of skin lesions were identified upon physical examination (Fig. 1A). Complete blood work revealed leukocytosis (20.4 k/μL; reference interval [RI], 6.1 to 18.3 k/μL), neutrophilia (13.9 k/μL; RI, 3.6 to 12.5 k/μL), and hypercalcemia (14.2 mg/dL; RI, 9.3 to 12.1 mg/dL). Thoracic and abdominal radiographs revealed irregular, thickened skin margins on the bilateral and ventral thoracoabdominal walls (Fig. 1B). On ultrasound, multiple cutaneous and subcutaneous lesions appeared diffuse and ill-marginated with heterogeneous echogenicity (Fig. 1C). In addition, irregular, heterogeneous enlarged bilateral medial iliac lymph nodes were identified.
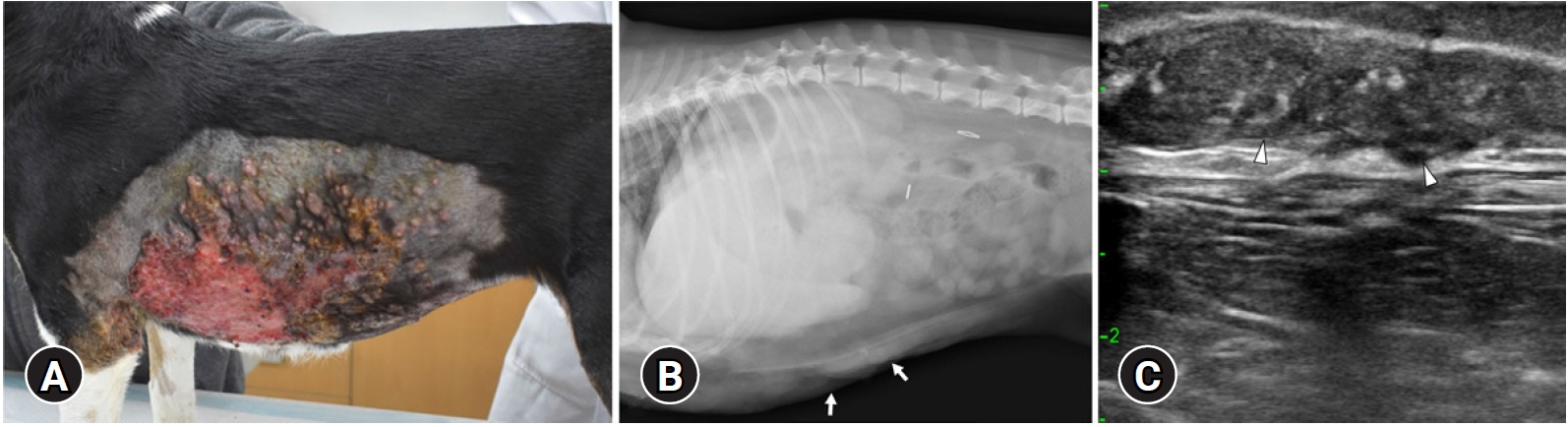
Photograph of the (A) skin lesion, (B) abdominal radiography, and (C) ultrasonography in a dog with cutaneous metastasis of mammary gland tumor. (A) Multiple skin lesions with crust and bloody exudates were identified on the flank. (B) Right lateral radiographs of the abdomen and (C) ultrasonographic images of the skin. Irregular, thickened skin margins on the bilateral and ventral thoracoabdominal walls are identified (arrows). The cutaneous and subcutaneous lesions showed diffuse, multiple, ill-marginated, and heterogeneous echogenicity (arrowheads).
CT examination was performed with a 32-multislice CT system (Alexion; Canon Medical Systems, Japan) for the precise evaluation of skin lesions and metastasis of MGT. The patient was positioned in dorsal recumbency on the CT table under general anesthesia. The scanning parameters were as follows: 120 kV, 150 mA, 3.0 mm slice thickness, and 0.75 seconds rotation times. A contrast study was performed after an intravenous administration of 600 mgI/kg iohexol (Bonorex 300 injection; Daehan Pharm, Korea) injected for 20 seconds using an autoinjector (A-60; Nemoto Kyorindo Co., Japan). Postcontrast CT images of the arterial, portal-venous, and delayed phases were obtained 20, 45, and 90 seconds after injection, respectively. Multifocal, irregular, and heterogeneously hyperattenuating thickened cutaneous and subcutaneous lesions were showed with heterogeneous contrast enhancement and partial calcification on the bilateral, ventral thoracoabdominal and left inguinal regions (Fig. 2A and B). In addition, enlarged, irregular and ovoid-shaped bilateral medial iliac and bilateral axillary lymph nodes with heterogeneous contrast enhancement were observed (Fig. 2C). No evidence of pulmonary metastasis was observed. The differential diagnosis for skin lesions included recurrent MGT, metastasis of MGT at the skin, or cutaneous inflammation with lymphadenopathy. Lesions in the left inguinal region were suspected to be a residual inguinal lymph node or granulomatous tissue due to previous surgery.
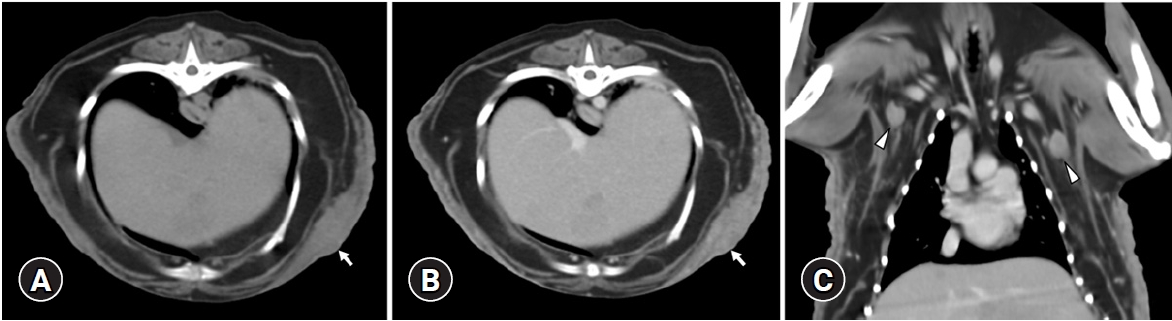
(A) Noncontrast and (B) postcontrast transverse abdominal computed tomography images and (C) postcontrast dorsal plane image. Multifocal, irregular, and heterogeneously hyperattenuating thickening with heterogeneous contrast enhancement cutaneous and subcutaneous lesions on the bilateral and ventral thoracoabdominal walls were identified (arrows). Enlarged axillary lymph nodes (arrowheads) were observed.
A skin lesion biopsy was performed. Histopathology revealed a well-demarcated, encapsulated, and densely cellular neoplasm composed of polygonal cells arranged in tubules and islands with necrotic centers supported by collagenous stroma. Anisocytosis marked with apoptosis was also detected in the neoplastic cells with distinct cell borders and eosinophilic cytoplasm containing frequent intracytoplasmic vacuoles, some consistent with signet ring cells. Furthermore, irregularly round nuclei with prominent nucleoli and stippled chromatin were found (Fig. 3). Based on the histomorphology of neoplasms with incomplete lymphatic invasion and history of MGT, a diagnosis of highly suspicious metastatic adenocarcinoma was made. The patient was scheduled for chemotherapy protocol with mitoxantrone (Mitron injection; Reyon Pharm, Korea) at 4 mg/m2 (intravenous) every 3 weeks for 5 treatments. After the first administration, improvement in skin lesions was observed; however, the patient died before the second induction due to dyspnea associated with pleural effusion, which we suspected was caused by MGT metastasis to the pleura, 1 month after the diagnosis.
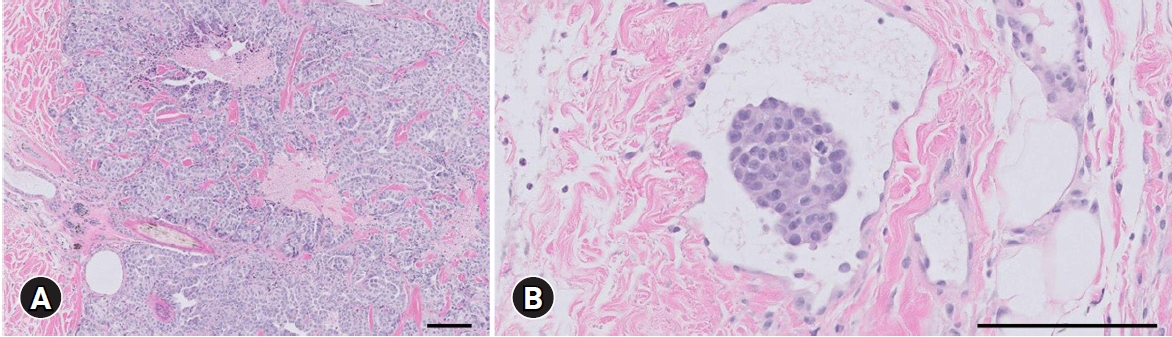
Histopathological section of the skin lesion. (A) A well-demarcated, encapsulated, and dense cellular neoplasm composed of polygonal cells arranged in tubules and islands with necrotic centers. (B) Incomplete obstruction of the lymphatic vessel with neoplastic cells. H&E, scale bars: (A) 100 μm, (B) 400 μm.
Skin metastases from visceral malignancies, except for hemangiosarcomas, are uncommon manifestations, accounting for 1% to 5% of all cutaneous lesions [4,8]. In particular, skin metastasis in dogs with MGT has been rarely reported. There have been only 4 cases in dogs; among them, 3 were reported without a description of clinical presentations [4-6]. Only one of these studies revealed the clinical presentation and histopathological findings. Painless, firm, round, brown, and multiple skin lesions were observed without ulcerations 2 years after the onset of MGT [4]. Conversely, the patient in this case report presented clinically different skin patterns from the previous study. Suppurative skin lesions were identified accompanied with pain and spread out within a week after the first operation. This study described not only the clinical symptoms but also diagnostic imaging and histopathological findings, which will be useful in veterinary clinics.
Cutaneous metastasis of MGT should be differentiated from that of inflammatory carcinoma because the clinical presentation of skin lesions is similar. Inflammatory carcinoma has a poor prognosis with a mean survival of 25 days and systemic illness [1,3]. Inflammatory carcinoma is a poorly differentiated carcinoma with widespread dermal lymphatic obstruction, characterized by extensive inflammation of the diffuse involvement of mammary glands with edema, warmth, and pain [2,3,9]. Dermal lymphatic involvement is considered a hallmark for the pathologic diagnosis of inflammatory carcinoma [10]. Metastasis to the bladder and reproductive organs rather than to the lungs, liver, and kidneys was more commonly observed than noninflammatory MGT [7]. Although differences between inflammatory carcinoma and cutaneous metastasis of MGT have not been fully elucidated, several differences are suspected. In the present case, skin lesions presented like those of inflammatory carcinoma; however, warmth and edema of the mammary glands, which are typical characteristics of inflammatory carcinoma, were not identified. In addition, upon histopathology, the lymphatic invasion was not as severe, and the cells were not as atypical or plugging the lymphatics, as is expected with the inflammatory carcinoma. Physical and histopathological examinations and evaluation of metastasized organs help differentiate inflammatory carcinoma from cutaneous metastasis of MGT.
Demonstrating the metastasis of the sentinel lymph nodes receiving the first lymphatic flow of MGT has an important prognostic value. Therefore, the regional lymph nodes must be evaluated in patients with MGT [1,11]. Diagnostic imaging including ultrasound and CT is useful to detect metastasis to the abdominal viscera or lymph nodes and to evaluate the degree of tumor invasiveness [3]. In particular, indirect CT lymphography, using an injection of contrast agent into the mammary parenchyma and identifying the sentinel lymph node help analyze the enhancement pattern of the lymph nodes and diagnose the presence of metastasis [11]. Heterogenicity or absence of sentinel lymph node opacification is associated with metastatic invasion [1,11,12]. The enhancement pattern of the lymph nodes was considered similar between indirect CT lymphography [11] and conventional contrast-enhanced CT [13]. In this study, postcontrast CT images showed heterogeneous enhancement of the enlarged bilateral axillary lymph nodes, and lymph nodes metastasis was highly suspected.
A limitation of this study was that sampling for histopathological examination was performed only on skin lesions. For the definite diagnosis, metastasis should show histopathological similarities to the primary tumor to determine whether the primary tumor is of origin [14]. However, in this study, the definitive diagnosis of lesions originating from the mammary gland was confirmed by the correlation between histopathological findings with evidence of lymphatic invasion and history of MGT.
In summary, skin metastasis from MGT rarely occurs in dogs. This study described the clinical manifestation, diagnostic imaging findings, treatment, and prognosis in a dog with cutaneous metastasis of MGT and suggests the possibility that various forms of skin metastasis may exist. Whenever patients with MGT accompanying cutaneous lesions, cutaneous metastasis of MGT should be considered as a differential diagnosis.
Notes
The authors declare no conflict of interest.
