Antiviral effect of 18-mer-peptide (1b-4/21-C12) on Japanese encephalitis virus and Akabane virus
Article information
Abstract
Japanese encephalitis virus (JEV) and Akabane virus (AKAV) are mosquito-borne viruses that cause encephalitis and reproductive disorders in horses and cattle, respectively. There is no treatment for JEV or AKAV infections in animals. Therefore, we evaluated the antiviral activity of 18-mer amphipathic peptides in the 1b-4/21-C series on JEV and AKAV using Vero cells in vitro and evaluated their effects on JEV in mice. Of 6 peptides, 1b-4/21-C12 had the lowest IC50 of 0.313 against JEV and its use as an antiviral against JEV and AKAV was examined. The IC50 of 1b-4/21-C12 against JEV and AKAV was 0.78 and 1.14 μM, respectively. Mice treated with 5 or 2 mg/kg of 1b-4/21-C12 had 32% and 16% survival rates, respectively, and the surviving mice treated with 1b-4/21-C12 began to gain weight beginning 8 days post challenge with the virulent Nakayama strain. Moreover, 20 μM 1b-4/21-C peptide had no cytotoxic effects on Vero cells. Our in vitro and in vivo results indicate that 1b-4/21-C12 has antiviral activity against enveloped JEV and AKAV and might be useful as a therapeutic substance.
Introduction
Japanese encephalitis virus (JEV) is an arthropod-borne pathogen in the family Flaviviridae transmitted to humans, horses, and pigs via mosquitoes. JEV causes acute encephalitis in humans and horses and reproductive disorders such as abortions and stillbirths in sow. Annually, 67,000 human JEV infections are reported in Asia and an abrupt increase in patients with JEV encephalitis was reported in South Korea in 2010 [1,2]. Inactivated and live-attenuated JEV vaccines have been developed for humans and animals and are currently used in South Korea, which has reduced the incidence of JEV infection in both humans and animals [3].
Akabane virus (AKAV) is mosquito-borne virus in the family Bunyaviridae that causes congenital central nervous system abnormalities in cattle, sheep, and goats. AKAV infections have been reported in Australia, Israel, Japan, China, and South Korea [4–6]. In South Korea, many AKAV infections occurred in 4 to 72-month-old cattle in 2010 [7]. Inactivated and live-attenuated AKAV vaccines have been developed for cattle and have helped to prevent AKAV infections since 2011 [8]. However, there are no effective drugs for patients or animals infected with JEV or AKAV.
Many substances have been investigated for use as antiviral drugs for JEV infections, such as specific RNA aptamers, single chain variable fragment (scFv) antibodies, antiviral bioflavonoids, N-methylisatin-β-thiosemicarbazone derivatives, ribavirin, and the lead compound CW-33 [9–11]. Those substances that inhibit virus replication or interfere with virus entry are effective at blocking viral infections. Compound CW-33 exerts antiviral activity against JEV by suppressing viral protein expression and genome synthesis [12]. Recently, peptides with antiviral activities have received much attention because of their high safety and low cost. Specific peptides can inhibit the proliferation of dengue virus (DENV), West Nile virus (WNV), Zika virus (ZIKV), and JEV infections in Vero cells [13,14]. Anti-HIV C-peptide (SJ-2176) and enfuvirtide are antiviral drugs for human immunodeficiency virus-1 (HIV-1) [15]. A brain-penetrating antiviral 27-amino-acid peptide is used to treat ZIKV infections [16]. These antiviral peptides can cross the blood–brain barrier and reduce viral infectivity by destabilizing the viral lipid envelope. Peptides are also less expensive to synthesize than other antiviral substances. Currently, no antiviral peptide for JEV or AKAV is available in South Korea.
In this study, we synthesized 6 peptides showing antiviral activity against JEV in vitro and further examined the antiviral activity of lb-4/21-C12 against JEV and AKAV in vitro and its efficacy against JEV in mice. The results provide a basis for developing antiviral peptides to treat JEV and AKAV infections.
Materials and Methods
Peptides
Peptides were synthesized using standard sold-phase synthesis and purified by chromatography (Anygen, Korea). The peptide sequence was NH2-WLRDVWDWICTVLTDFKT-COOH (Fig. 1). Fatty acids were conjugated to the N-terminal end of the peptide using the spacer γ-Glu-OEG-OEG, and the peptides were named 1b-4/21-C0, 1b-4/21-C8, 1b-4/21-C10, 1b-4/21-C21, 1b-4/21-C14, and 1b-4/21-C16 according to the fatty acid carbon chain. For the in vitro experiment, the 1b-4/21-C peptides were solubilized in dimethyl sulfoxide to 1 mg/mL and used as a stock solution and then stored at -20℃ until use. The peptides were diluted with Dulbecco’s modified Eagle’s medium (DMEM) for antiviral tests and cytotoxicity in vitro.
Cells and viruses
Vero (ATCC CCL-81, USA) cells were grown in DMEM with 10% heat-inactivated fetal bovine serum (FBS) and antibiotic-antimycotic (Gibco BRL, USA). The Vero cells were used to propagate JEV and AKAV. JEV strain KV1899 (GenBank acc. no. AY316157), which was isolated from pig’s blood in 1999, was used to examine the antiviral effect. The AKAV strain KV0505 (GenBank acc. no. DQ973188) was isolated from blood of naturally infected Korean cattle in 2005.
Propagation and virus titration
Vero cells were inoculated with JEV and AKAV and the virus was harvested 5 days post-inoculation. After harvesting the virus, the titers of the 2 viruses were determined based on the presence of typical cytopathic effects (CPEs) by diluting 100 μL of each virus 10-fold in 96-well microplates and adding 100 μL of fresh DMEM containing 2 × 105 Vero cells. The Vero cells were observed for CPEs 5 days post-inoculation. Viral titers measured by the Reed–Muench method were expressed as the median tissue culture infectious dose per milliliter (TCID50/mL) [17].
Cell cytotoxicity assay
The effect of 1b-4/21-C peptide treatment on the viability of Vero cells was determined using serial dilutions of 1b-4/21-C peptide (40-0.312 μM in DMEM). Vero cells were seeded and incubated with the peptides for 5 days. Cell status was observed under a microscope.
Viral reduction assay
Viral reduction tests for JEV and AKAV were performed in 96-well microplates using Vero cells. A 100-μL aliquot of 1b-4/21-C12 at 6 concentrations (0.15, 0.31, 0.62, 1.25, 2.5, and 5 μM) was mixed with an equal volume of JEV or AKAV containing 103.0 or 103.5 TCID50/0.1 mL and incubated at 37℃ for 1 h. Each mixture was diluted 10-fold and aliquoted into 96-well microplates. Vero cells (100 μL in DMEM containing 5% FBS, n = 20,000 cells/well) were added to each well and the microplates were incubated for 5 days at 37℃ in a 5% CO2 incubator, and virus-induced CPEs were evaluated under a microscope. The viral titers were expressed as the reciprocal of the highest dilution that completely inhibited viral CPEs. The viral titers of JEV or AKAV in culture medium without 1b-4/21-C12 were used as a positive control. Vero cells grown without 1b-4/21-C12 and viruses were used as negative controls. All tests were repeated twice or more. The 50% inhibitory concentration (IC50) was calculated by nonlinear regression using GraphPad Prism 5 (GraphPad, USA).
Antiviral effects of 1b-4/21-C12 in mice
The mouse JEV infection experiment followed the laboratory animal ethics committee protocol and was approved by our institutional Laboratory Animal Care and Use Committee at the Animal and Plant Quarantine Agency (no. 2021-568). Three-week-old mice were divided randomly into 4 groups of 6 mice each. For infection, groups 1 to 3 were inoculated peritoneally with 10 LD50 of JEV, Nakayama strain. For treatment, the mice in groups 1 and 2 were given 5 and 2 mg/kg of 1b-4/21-C12 intraperitoneally 24 and 48 hours after virulent JEV inoculation. The clinical conditions and body weights of the mice were assessed daily for 14 days. The mouse survival data were analyzed using the log-rank test.
Results
Selection of 1b-4/21-C
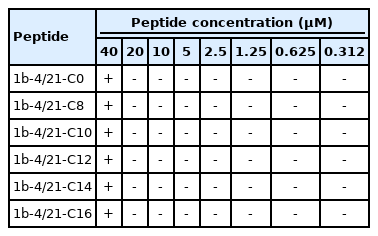
As the fatty acids conjugated to 1b-4/21-C peptide might affect antiviral activity, we assessed the inhibition of JEV infection by 1b-4/21-C0, -C8, -C10, -C12, -C14, and -C16 peptides in Vero cells. Solutions of each peptide were incubated with JEV for 1 hour at 37°C, and then used to infect Vero cells. Viral titration demonstrated that 1b-4/21-C12 had the lowest IC50 (0.313 μM) of JEV among the 6 peptides (Fig. 2). Therefore, 1b-4/21-C12 was selected for use as an antiviral against JEV and AKAV.

Viral reduction assay for 6 peptides exhibiting antiviral activity against Japanese encephalitis virus in Vero cells. Viral titers of antiviral activity of peptides were repeated twice and the results were expressed as average value. The number after 1b-4/21-C is the number of carbons in the fatty acid.
Antiviral activity of 1b-4/21-C12 peptide against JEV and AKAV infections in vitro
To determine the IC50 of 1b-4/21-C12 against JEV and AKAV, the viruses at titers of about 103.5 TCID50/mL were incubated with 1b-4/21-C12 at concentrations ranging from 0 to 5 μM. Then, the titers of 2 viruses were measured in Vero cells. As shown in Fig. 3, 5 μM 1b-4/21-C12 significantly reduced JEV- or AKAV-induced CPEs in Vero cells, with IC50’s against JEV and AKAV of 0.78 and 1.14 μM, respectively.
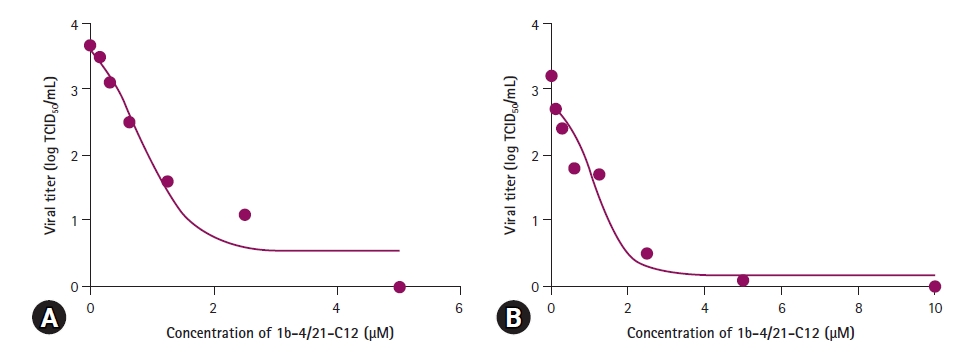
1b-4/21-C12 peptide shows antiviral activity against (A) Japanese encephalitis virus (JEV) and (B) Akabane virus (AKAV) in vitro. Viral titers of antiviral activity of 1b-4/21-C12 against JEV and AKAV were repeated twice and the results were expressed as average value. At 5.0 μM, 1b-4/21-C12 completely inhibited JEV and AKAV.
In vivo efficacy of 1b-4/21-C12 in mice
Since 1b-4/21-C12 significantly inhibited JEV infection in Vero cells and had an IC50 of 0.78 μM against JEV in vitro, we performed an efficacy test to see if 1b-4/21-C12 protects mice from a lethal JEV infection. Two groups of mice challenged with JEV peritoneally were inoculated with 5 or 2 mg/kg of 1b-4/21-C12 via the same route and observed for 14 days. Mice started to die beginning 5 days post challenge. Groups 1 and 2 mice treated with 5 and 2 mg/kg of 1b-4/21-C12, respectively, had survival rates of 32% and 16% compared to 0% for the control mice (Fig. 4A). We also examined whether 1b-4/21-C12 protected mice after intracranial JEV infection using the same doses of 1b-4/21-C12; in this case, however, 1b-4/21-C12 failed to protect the mice, which had 0% survival rates (data not shown).

The peptide 1b-4/21-C12 has antiviral activity against Japanese encephalitis virus in vivo. Mice inoculated twice with 5 or 2 mg/kg of 1b-4/21-C12 had respective survival rates (A) of 32% and 16%. The mean body weights (B) of 4 groups (n = 6) of mice were measured for 14 days.
All mice were weighed daily. The group 1 and group 2 mice inoculated with JEV plus 5 or 2 mg/kg of 1b-4/21-C12 showed marked weight loss from 5 to 7 days post challenge and then the weights of the surviving mice began to increase (Fig. 4B).
Cytotoxicity
As shown in Table 1, none of the 6 peptides was cytotoxic at a concentration of 20 μM. However, Vero cells cultured in DMEM containing 40 μM 1b-4/21-C peptide showed cytotoxicity beginning 2 days after exposure.
Discussion
Despite the low incidence of Japanese encephalitis, approximately 30% of severe patients die. There is no specific treatment for JEV infection in humans or animals. Therefore, it is necessary to develop agents to treat JEV infection. Peptides derived from various sources, such as JEV E protein, phage display libraries, and synthetic materials, inhibit infections caused by ZIKV, JEV, WNV, DENV, and HIV-1 [13–16]. No peptides showing antiviral activity against JEV and AKAV have been reported in South Korea. In this study, we synthesized six 18-amino-acid peptides and evaluated their antiviral activities. Peptide 1b-4/21-C was conjugated with fatty acids to improve its stability. Of the 6 peptides tested, 1b-4/21-C12 significantly inhibited JEV infection in Vero cells. Peptides such as 1b-4/21-C12 inactivate viral particles by disrupting the integrity of the relatively small viral membrane [18]. Based on the results of selection tests, we measure the IC50 of peptide 1b-4/21-C12 against JEV and AKAV in vitro.
Antiviral peptides such as 1b-4/21-C12 are easy and economical to synthesize. We proved that 1b-4/21-C12 has antiviral activity against JEV and AKAV in vitro with a respective IC50 of 0.78 and 1.14 μM, respectively. The IC50 of 1b-4/21-C12 for JEV was 46-fold lower than that (35.9 μM) of phage display peptide (P1) and 5.7-fold higher than that (0.136 μM) of a 27-mer amphipathic peptide [14,16]. The IC50 of 1b-4/21-C12 against AKAV was similar to that (1.3 μM) of hexachlorophene, a drug approved by the Food and Drug Administration (FDA) [19], suggesting that 1b-4/21-C12 may have antiviral activity against other viruses, such as ZIKV, DENV, WNV, and Yellow fever virus. It is thought that1b-4/21-C12 exerts antiviral activity by attaching to and destroying the viral envelope in a similar way to that of the 27-mer amphipathic peptide [16].
In the efficacy tests, we found that mice inoculated with 5 and 2 mg/kg of 1b-4/21-C12 had respective survival rates of 32% and 16%. The survival rate against JEV with 1b-4/21-C12 treatment is relatively low compared to the 70% in mice inoculated with phage display peptide or JEV E stem-derived peptide [13,14]. The efficacy of our peptide might be enhanced on combination with ribavirin, scFv, and FDA-approved drugs [20–22]. Future studies should measure the half-lives of peptides conjugated with various fatty acids and determine the exact mechanism of action of 1b-4/21-C.
In conclusion, we demonstrated that the 18-mer peptide 1b-4/21-C12 had in vitro antiviral activity against JEV and AKAV, which possess lipid envelopes. 1b-4/21-C12 also showed antiviral efficacy against JEV in mice and was not toxic to Vero cells at 20 μM. The antiviral activities against the 2 viruses should prompt broader efforts to design new strategies for treating viral infections in animals.
Notes
The authors declare no conflict of interest.
Acknowledgements
This work was supported financially by a grant (121005-1) from Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry (IPET), Ministry of Agriculture, Food and Rural Affairs (MAFRA), Republic of Korea.