Incidence and sero-surveillance of feline viruses in Korean cats residing in Gyeonggi-do
Article information
Abstract
Incidences of major feline viral diseases provide basic information for preventing viral disease in cats. Despite the growing interest in feline viral diseases, sero-surveillances have been lacking. In this study, we analyzed the diagnoses of feline viral diseases and conducted a sero surveillance of feline panleukopenia virus (FPV), feline calicivirus (FCV), feline herpesvirus-1 (FHV-1), and feline infectious peritonitis virus (FIPV) in Korean cats. Of the 204 confirmed cases since 2015, the numbers of diagnoses for FPV, FIPV, FCV, feline influenza virus, and FHV-1 were 156, 32, 12, 3, and 1 case, respectively. In total, 200 sera, collected between 2019 and 2021, were screened for the presence of antibodies against FPV, 2 FCVs, FHV-1, and FIPV using a hemagglutination inhibition test and a virus-neutralizing assay (VNA). The overall seropositive rates in cats tested for FPV, the 2 FCVs, FHV-1, and FIPV were 92.5%. 42.0%, 37.0%, 52.0%, and 14.0%, respectively. A low correlation (r = 0.466) was detected between the VNA titers of 2 FCV strains. The highest incidence and seropositive rate of FPV reveal that FPV is circulating in Korean cats. The low r-value between 2 FCVs suggests that a new feline vaccine containing the 2 kinds of FCVs is required.
Introduction
Feline panleukopenia virus (FPV), feline calicivirus (FCV), feline herpesvirus-1 (FHV-1), and feline coronavirus (FCoV) are major infectious pathogens in cats worldwide [1–4]. FPV (family Parvoviridae) is highly related to canine parvovirus type 2, with 98% or more homology [5]. FPV transmitted via the oronasal and fecal routes has been reported in Felidae and wild animals [6]. The clinical symptoms of FPV infection in cats include high fever, anorexia, vomiting, and hemorrhagic diarrhea resulting in death in about 50% of infected kittens [7]. FPV is very stable in the environment and causes persistent infection. The FPV VP2 protein induces neutralizing antibodies and has been used to detect FPV antibodies after natural infections and vaccination [8].
FCV (genus Vesivirus, family Caliciviridae) is a highly adaptable RNA virus that evolves rapidly under harsh environmental pressure [2]. FCV is transmitted via the oronasal route, and ocular discharge from infected cats is highly contagious to other cats. FCV infection manifests in 2 forms in cats < 1 year of age. The clinical signs known as the classic form are fever, nasal and ocular discharge, oral ulcerations, and conjunctivitis. The clinical symptoms known as virulent systemic disease are pyrexia, cutaneous edema, ulcerative dermatitis, anorexia, and jaundice with sudden death [2,9]. The VP1 protein containing the major B cell epitopes acts as a target for inducing the neutralizing antibody against FCV [10].
FHV-1 (genus Varicellovirus, subfamily Alphaherpesvirinae, family Herpesviridae) consists of approximately 135 kbp and causes feline viral rhinotracheitis. FHV-1 is transmitted through contact with a recently infected cat via the oronasal route [11]. Cats infected with FHV-1 develop fever, nasal discharge, conjunctivitis, keratitis, and pneumonia, and may die [12]. Infection of the trigeminal ganglion by FHV-1 leads to a latent infection and clinical signs can be reactivated by high stress or immunosuppression, resulting in infectious virus shedding [13]. Glycoproteins known as gB, gC, and gD play a key role in the induction of the humoral and cell-mediated immune responses [14].
FCoV (family Coronaviridae) has different antigenic relevance to canine coronavirus. Based on a serological and molecular analysis, FCoV is classified into 2 biotypes called feline enteric coronavirus (FECV) and feline infectious peritonitis virus (FIPV) [4]. FECV infection shows mild or subclinical enteric symptoms, but cats infected with FIPV develop clinical signs, such as lethargy, decreased appetite, weight loss, and eventually death [15]. FIPV is transmitted from infected cats via the fecal or oral route.
Methods such as virus neutralization (VN), hemagglutination inhibition (HI), enzyme-linked immunosorbent assays, and one-step immune chromatography assays are used to detect antibodies against FPV, FCV, FHV-1, and FIPV [16,17]. Among these serological methods, the HI test to detect FPV antibodies has been used as a standard method, and the VN test has been used to measure antibodies against FCV, FHV-1, and FIPV. Although FPV, FCV, FHV-1, and FIPV infections have been reported in South Korean cats [17–20], a recent incidence and serologic investigations of major feline pathogens are lacking. In this study, we analyzed the incidence of feline viral diseases since 2015. Serological investigations of FPV, the 2 FCVs, FHV-1, and FIPV were performed using 200 cat sera collected between 2019 and 2021.
Materials and Methods
Cells and viruses
CRFK (CCL-94; ATCC, USA) cells were grown in Dulbecco’s modified Eagle medium supplemented with 10% (v/v) heat-inactivated fetal bovine serum and an antibiotic-antimycotic solution (Gibco, USA). The cells were used to propagate FPV, the 2 FCVs, FHV-1, and FIPV, and for the virus neutralization assay (VNA). The FPV1901, FCV17D03, FCV17D283, and FHV191071 strains were isolated from naturally infected Korean cats in 2017 and 2019, respectively [17–19]. The FIPV strain was obtained from a commercial live FIPV vaccine.
Blood sampling
A total of 200 blood samples were collected for the sero-surveillance study from cats residing in Gyeonggi Province, South Korea from 2019 to 2021. The age of cats varied from 0.5 to 10 years. Among the cat breeds, Korean domestic short hair was the most common. No information was available about whether the cats had been inoculated with vaccines. Blood samples were subjected to centrifugation and the sera were stored at -20°C until use.
HA and HI tests
The HA test was performed by preparing serial 2-fold dilutions of the FPV strain in 50 µL of Sorensen buffer (pH 6.0) with 50 µL of 0.6% pig erythrocytes. The HA titer was expressed as the reciprocal of the highest dilution of FPV that hemagglutinated. The HI test of the FPV1901 strain was performed in a 96-well microplate as described previously [1], with slight modifications. Briefly, 50 µL of serum were treated with 25% kaolin and packed pig erythrocytes to remove non-specific inhibitors. Eight HA units of FPV1901 in 25 µL were added to 25 µL of the treated serum for the HI test. After 1 hour of incubation at 37°C, 50 µL of 0.6% pig erythrocytes were added, and the microplates were incubated at 4°C for 1 hour. The HI titer of the cat serum was expressed as the reciprocal of the highest dilution of serum showing complete inhibition of hemagglutination. Serum samples with HI titers ≥ 1:20 were considered positive [21].
Virus neutralization assay
The VNA for FCV17D03, FCV17D283, FHV191071, and FIPV was carried out in 96-well plates using cat sera inactivated at 56°C for 30 min. Fifty microliters of 2-fold serially diluted serum were mixed with an equal volume of FCV17D03, FCV17D283, FHV191071, or FIPV containing 200 TCID50/0.1 mL. After incubating the mixture at 37°C for 1 hour, 100 μL of a CRFK cell suspension containing 2 × 104 cells were added to each well. The plates were incubated for 3 to 4 days in a humidified incubator with 5% CO2. Each well was examined under a microscope to detect virus-specific cytopathic effects (CPEs). The VNA titers were expressed as the reciprocal of the highest serum dilution showing complete inhibition of the CPEs. A VNA titer ≥ 1:2 was considered positive against FCV17D03, FCV17D283, FHV191071, and FIPV.
Statistical analysis
Least-squares linear regression analysis was employed to determine the correlation coefficients (r values) between the VNA titers of the 2 FCV strains. The r values were automatically calculated in Microsoft Excel 2010 software (Microsoft Corp., USA). A p-value < 0.05 was considered significant.
Results
Feline viral pathogens, including FPV, FCV, FHV-1, feline influenza virus (FinV), and FIPV, were investigated using data from the Animal and Plant Quarantine Agency (APQA) in South Korea from 2015 to 2021. As shown in Fig. 1, of the 204 confirmed cases, the largest number of diagnoses was confirmed as FPV (156 cases). The numbers of diagnoses for FIPV, FCV, FinV, and FHV-1 were 32, 12, 3, and 1 case, respectively. No infections of the feline leukemia virus and feline immunodeficiency virus were diagnosed.

Numbers of feline viral infections confirmed by the Animal and Plant Quarantine Agency from 2015 to 2021 (www.kahis.go.kr). A total of 468 cat samples were received, and 204 cases were diagnosed with a viral infection. FPV, feline panleukopenia virus; FIPV, feline infectious peritonitis virus; FCV, feline calicivirus; FinV, feline influenza virus; FHV-1, feline herpesvirus-1.
The seroprevalence rates of FPV, the 2 FCVs, FHV-1, and FIPV using the HI and VNA results were examined in 200 cat sera collected from Gyeonggi Province South Korea. Of the 200 cats tested in this study, 109 were males and 91 were females. The mean age of the cats was 2.4 years. The overall seropositive rates for FPV, FCV17D03, FCV17D283, FHV-1, and FIPV were 92.5% (185/200), 42.0% (84/200), 37.0% (74/200), 52.0% (104/200), and 13.5% (27/200), respectively (Table 1). No significant differences in the seropositive rates were observed according to year or gender in the 5 viruses.

Seropositive rates of FPV, FCV, FHV-1, and FIPV using hemagglutination inhibition and virus neutralization assays in feline blood samples collected from Gyeonggi Province, South Korea
An HI titer ≥ 1:20 and a VNA titer ≥ 1:2 were considered to be seropositive against FPV, FCV17D03, FCV17D283, FHV-1, and FIPV. About 64.5% (129/200) of the HI titers against FPV were > 1:80, indicating a protective antibody titer. The most frequent HI titer was 1:160 (17.5%). The most frequent VNA titers against the 2 FCVs and FHV-1 were 1:2 (13.5% and 12.0%) and 1:4 (11.0%), respectively (Fig. 2). No significant differences in the seropositive rates for FPV were detected in the distribution of the HI titers according to age. However, cats > 7 years of age had the highest seropositive rates (65.2% and 30.4%) for FCV17D03 and FIPV. Five-year-old cats had the highest seropositive rate (83.3%) against FHV-1 (Fig. 3).

Distributions of hemagglutination inhibition (HI) titers and virus neutralization (VN) titers against (A) feline panleukopenia virus (FPV), (B) feline calicivirus (FCV), (C) feline herpesvirus-1 (FHV-1), and (D) feline infectious peritonitis virus (FIPV) in 200 cat sera. An HI titer of 1:20 and a VN titer of 1:2 or higher were considered seropositive against the 5 feline viral pathogens.
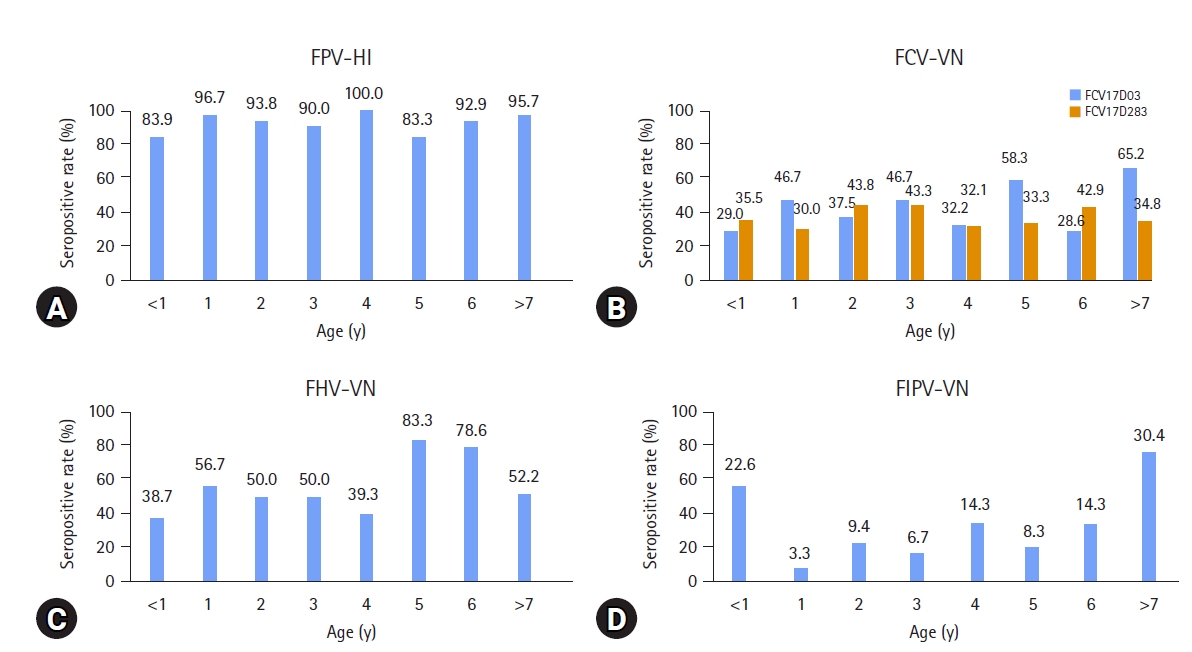
Seropositive rates of the hemagglutination inhibition (HI) titers and virus neutralization (VN) titers against (A) feline panleukopenia virus (FPV), (B) feline calicivirus (FCV), (C) feline herpesvirus-1 (FHV-1), and (D) feline infectious peritonitis virus (FIPV) according to age. Cats of all ages had a high seropositive rate for FPV.
Figure 4 shows the regression lines and correlation coefficients (r) between the VNA titers and the 2 FCV strains. The r-value was 0.466, indicating a significant difference between the VNA titers of the FCV17D03 and FCV17D283 strains.
Discussion
A total of 468 cat samples have been sent to the APQA for diagnosis since 2015, and 204 cases were confirmed by histopathological and molecular methods. In our study, 76.4% (156/204) of cats diagnosed at the APQA were confirmed to have FPV, indicating that FPV is the most lethal pathogen causing death in Korean cats. Second, 15.7% (32/204) of the cats were diagnosed with FIPV, which is fatal [15]. Infections of FCV, FinV, and FHV-1 were also identified, and these diseases have been reported worldwide [2,3,22]. Diagnostic information reveals which viral pathogens are circulating in the cat population and provides basic data for prevention.
Sero-surveillance has been used as part of disease management for animal populations. Data obtained through serologic surveys of FPV, FCV, FHV-1, and FIPV help to understand the immune or infectious status in the cat population. In addition, these data provide a basis for recommending vaccination to prevent FPV, FCV, FHV-1, and FIPV. Our seroprevalence results suggest that recommending vaccinations for stray cats is a possible solution [23].
FPV infection has been reported in many countries, and the seroprevalence of FPV has been estimated to range from 28.0% to 83.9% depending on the country and time [23,24]. Anti-FCV, FHV-1, and FIPV antibodies have also been reported in many countries but their levels are highly variable depending on the age and vaccination status of the cats [23–25]. Our serosurvey revealed that the overall seropositive rates against FPV, FCV17D03, FCV17D283, FHV-1, and FIPV were very high (92.5%) for FPV and moderately low (13.5%) for FIPV.
The high FPV seropositive rate suggests that Korean cats are continuously exposed to FPV in the field. Analysis of serum antibody levels helps to determine whether cats can protect themselves against re-exposure to a specific pathogen. It has been reported that dogs with HI titers ≥ 1:80 are protected against a canine parvovirus challenge [26]. Therefore, we set and analyzed an HI titer of ≥ 1:80 as a protective antibody titer. Our study showed that 64.5% of the cats tested had HI titers ≥ 1:80, indicating that 35.5% of the cats are not protected against wild FPV infection and require vaccination.
In our study, serum antibodies were measured using the 2 FCVs and FHV-1 isolated from Korean cats because of the high genetic variation in FCV [27]. As FCV and FHV-1 are involved in humoral and cellular immunity, it is difficult to judge protection using the VNA titer alone. However, one way to determine whether a cat has been exposed to FCV or FHV-1 is to measure the VNA titer. Our seropositive rates for the 2 FCVs, FHV-1, and FIPV were 42%, 37%, 52%, and 14%, respectively, which are similar to or lower than those reported in other countries [23,24]. Therefore, vaccination is recommended to protect cats from FCV and FHV-1 infection. FIPV has been accepted as an FCoV variant due to specific gene mutations [4]. Cats infected with FIPV and showing clinical symptoms usually die. Therefore, the FIPV antibody titer present in the cat’s serum is considered to be a sign of exposure to FIPV or a response to vaccination.
The cross-neutralization test has been used to demonstrate serological differences between mutated viruses or to evaluate the potency of a vaccine against a variant strain [28]. FCV has been divided into at least 2 genogroups based on the VP1 sequence [29]. In this study, the correlation value of the VNA performed using 2 FCVs with 84.8% genetic homology was 0.466, indicating that genetic diversity and serological differences may exist in FCV. Therefore, cats should be inoculated with an FCV vaccine containing at least 2 variants.
In conclusion, major feline viral diseases were investigated in South Korea. The most frequently detected virus in cats was FPV, followed by FIPV, FCV, FinV, and FHV-1. The overall seropositive rates in cats tested for FPV, FCV17D03, FCV19D283, FHV-1, and FIPV were 92.5%. 42.0%, 37.0%, 52.0%, and 14.0%, respectively, indicating that FPV causes persistent circulating infection in the Korean cat population. The serological correlation for the 2 FCV strains was moderately low (r = 0.466), suggesting that there are 2 FCV serotypes. The data presented in this study indicate that FPV is the most life-threatening pathogen in feline viral diseases, and has a high seropositive rate and there are 2 serotypes for FCV. Thus, more sophisticated vaccine program for cats should be considered. Additional studies on the efficacy of FPV vaccine are needed. Moreover, a new FCV vaccine should contain 2 types of FCV strains.
Notes
The authors declare no conflict of interest.
Acknowledgements
This study was supported financially by a grant (B-1543083-2019-21-01) from the Animal and Plant Quarantine Agency, Ministry of Agriculture, Food and Rural Affairs (MAFRA), Republic of Korea.

