Neuronal ceroid lipofuscinosis in a Border Collie: a case report
Article information
Abstract
A 2-year-old spayed female Border Collie presented with visual deficits and behavioral changes. Neurological examination revealed bilateral menace response deficit with a normal pupil light reflex. Cerebral cortical thinning, cerebral sulci and cerebellar fissure widening, ventriculomegaly, and cerebral atrophy were observed on magnetic resonance imaging (MRI). Histopathology revealed fluorescent lipopigment accumulation in the cerebrum, and the dog was diagnosed with neuronal ceroid lipofuscinosis. This is the first case report describing the changes in clinical signs, MRI findings, and histopathologic changes in neuronal ceroid lipofuscinosis in Korea.
Neuronal ceroid lipofuscinosis (NCL) is a group of inherited neurodegenerative lysosomal storage diseases in humans and animals and is characterized by apparently normal development followed by progressive deterioration in cognitive and motor functions, blindness, seizures, respiratory impairment, and premature death in most cases [1,2]. The diagnosis of NCL can be established through clinical signs, magnetic resonance imaging (MRI), histopathological findings, and gene sequencing used to probe mutated genes, including CLN10, CLN5, CLN12 and CLN8 [3,4]. However, gene sequencing is not generally conducted in veterinary medicine due to few studies on NCL diagnosis. Massive lysosomal accumulation in neurons and neuronal loss are reported to be representative pathological features of NCL [5]. Reported MRI lesions of the NCL include cerebral and cerebellar atrophy, mild hyperintensity of the cerebral white matter, cortical thinning, and thalami hypointensity on T2-weighted images [6]. Although several treatments, including enzyme replacement, stem cell and gene therapies, and pharmacological treatment, have been used to treat dogs with NCL, their prognosis is usually poor [7,8].
A 2-year-old spayed female Border Collie presented with loss of vision, as evidenced by her bumping into walls, and progressive behavioral changes including aggression toward the owner. Decreased response to food from the age of 5 months and descending stairs incorrectly from the age of 1 year were identified through history taking. In addition, aggressive behavior of trying to bite the owner accompanied by failure to recognize the owner was observed during the first visit. Mild increased intraocular pressure (right eye, 21 mmHg; left eye, 23 mmHg) and bilateral menace response deficit with normal pupil light and dazzle reflexes were identified through ocular examination. However, no abnormal findings were observed on electroretinography or examination of the optic fundus. No abnormalities were observed in the blood analysis. Bilateral menace response deficit was the only abnormal finding on neurological examination.
Based on these results, neurolocalization was observed in the forebrain, particularly in the occipital lobe. MRI (1.5-Tesla unit, Signa Creator; GE Healthcare, USA) was performed to confirm forebrain abnormalities, and generalized cerebral cortex thinning was observed. Mild-to-moderate dilation of the mesencephalic aqueduct and moderate ventriculomegaly of the third, fourth, and lateral ventricles were also observed (Fig. 1A). Ventriculomegaly was identified when the right and left ventricle-to-brain height ratios were 37.4% and 29.9%, respectively. Moderate dilated cerebral sulci were observed with hyperintensity on T2-weighted imaging and hypointensity on fluid-attenuated inversion recovery and T1-weighted imaging. The cerebellum was moderately small, and mild-to-moderate widening of the cerebellar fissure was observed on T2-weighted imaging (Fig. 1B). No abnormal findings were detected in the optic nerve or chiasm. Results of cerebrospinal fluid analysis, including cytology and polymerase chain reaction for infectious pathogens (Bartonella spp., Blastomyces dermatitidis, Coccidiosis spp., Cryptococcus spp., Histoplasma capsulatum, Canine distemper virus, West Nile virus, Borrelia burgdorferi, Neospora spp., and Toxoplasma gondii) were normal.

Representative magnetic resonance imaging of the forebrain atrophy of a Border Collie with neuronal ceroid lipofuscinosis. (A) Enlarged lateral and third ventricles (arrows) are identified in T2-weighted imaging of a transverse section at the level of the hypothalamus. (B) Small interthalamic adhesion (asterisk) and cerebellar sulci widening (arrowhead) are identified in T2-weighted imaging of the midsagittal section at the level of the interthalamic adhesion.
Prednisolone (Solondo; Yuhan, Korea) 0.5 mg/kg, furosemide (Lasix; Handok, Korea) 1 mg/kg, gabapentin (Neurontin; Pfizer, USA) 20 mg/kg twice daily, and omeprazole (Ramezole; Hanmi, Korea) 10 mg/kg once daily were administered orally to treat ventriculomegaly. The dog was euthanized with the owner’s consent despite a slight improvement in aggressive behavior after 2 weeks of treatment.
After necropsy was performed, the dog’s brain was fixed with 10% formalin and stained with hematoxylin and eosin (H&E) (Fig. 2A), autofluorescence (Fig. 2B), and periodic acid-Schiff (PAS) (Fig. 2C). Immunohistochemistry was performed using antibodies directed against lysosomal-associated membrane protein 1 (ab24170, LAMP-1; Abcam, UK) (Fig. 2D). H&E staining showed that neuronal cells comprised abundant bright intracytoplasmic storage materials. Some of the cytoplasm of the neurons was distended by storage products, and those compressing the nuclei were observed by H&E staining. A moderate decrease in the number of granular layers and Purkinje cells was observed. According to autofluorescence staining, neurons throughout the brain had abundant autofluorescent inclusions. Positive storage material in the cytoplasm of neurons stained variably with magenta with PAS. The cytoplasm of the neurons stained for LAMP-1. In summary, the dog was diagnosed with NCL based on these histopathological findings.
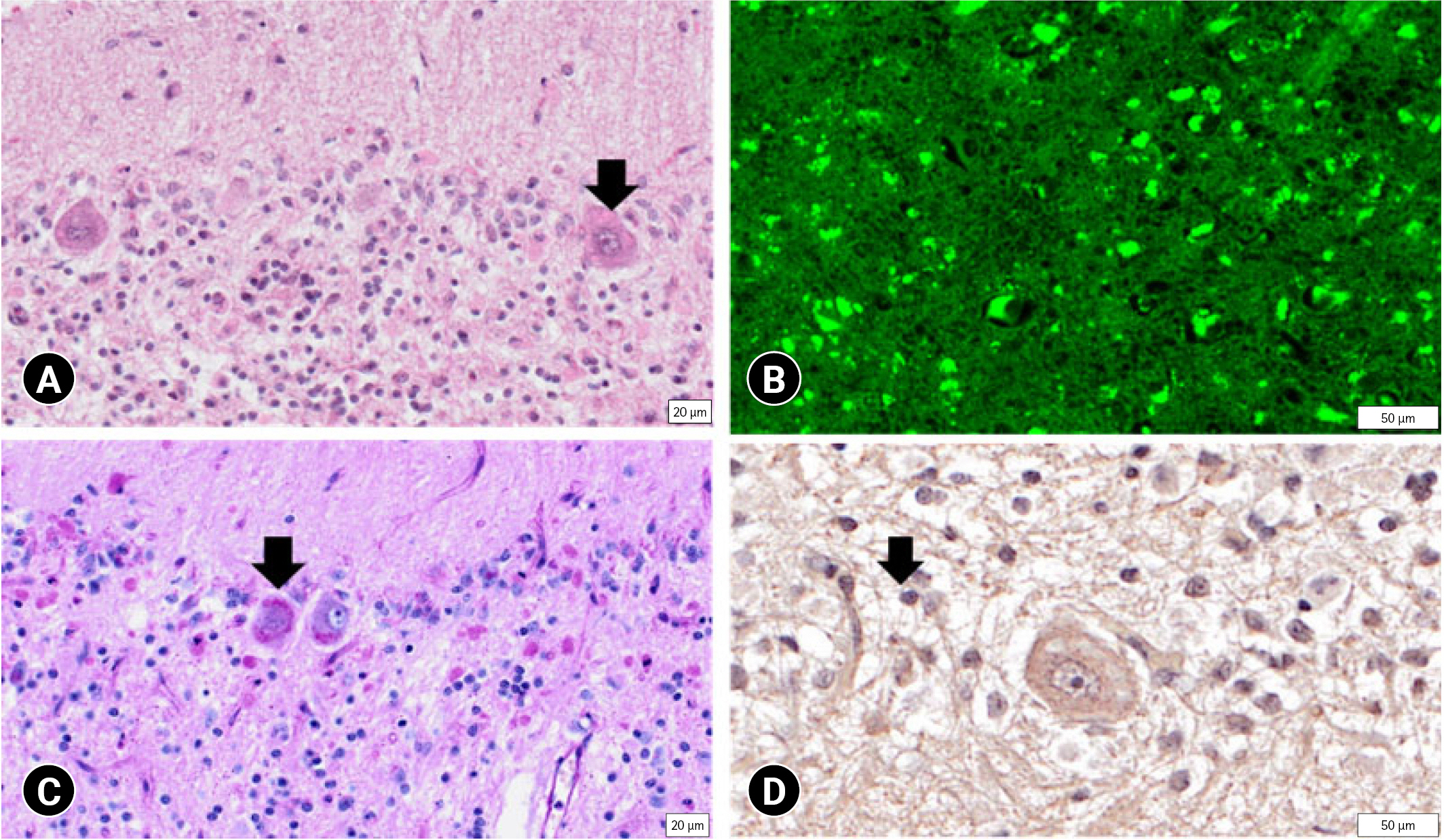
(A) Neuronal cells contain abundant bright intracytoplasmic storage materials (arrow) (hematoxylin and eosin). Bar = 20 μm. (B) Neurons throughout the brain have abundant autofluorescent inclusions (autofluorescence). Bar = 50 μm. (C) Positive storage material (arrow) in the cytoplasm of neurons (periodic acid-Schiff stain). Bar = 20 μm. (D) The cytoplasm of neurons shows staining against lysosomal-associated membrane protein 1 (arrow). Bar = 50 μm.
To the best of our knowledge, canine NCL has not yet been reported in Korea. The present report describes the clinical signs and MRI and histopathological findings of NCL in a Border Collie.
NCL is a disease that involves the accumulation of ceroid lipofuscin in the brain. Deposition of ceroid lipofuscin affects the cerebral and limbic systems [9]. Lesions in the frontal lobe and internal capsule projections result in unawareness of the owner, slow learning, and pacing. Behavioral changes may occur if the disease occurs due to organophosphorus deposition in the telencephalon or diencephalon [9]. Lesions in the temporal lobe cortex, limbic system, and hypothalamus can lead to aggression and bizarre behavior and feeding disorders [9]. Behavioral abnormalities, such as loss of interest in play, begin at 15 months of age but usually manifest a few months later (at approximately 18 months of age) at an early stage [10]. Visual disorders are unambiguous, and behavioral abnormalities increase in severity in the middle stage (19 to 23 months of age) [11]. Convulsive seizures and motor disorders occur at the late-to-terminal stages due to a wide spectrum of brain dysfunction (older than 22 months of age) [11].
NCL in a Border Collie was first defined in Australia in the 1980s, and a sporadic case of the disease was also reported in the USA in the 1990s [12]. Behavioral changes, motor abnormalities, and blindness were observed in the affected dogs. In this case, clinical symptoms, including aggressive behavior, movement impairment, and visual defects, started between 18 and 20 months of age. These findings indicate that this clinical form of NCL strongly resembles those previously reported in Australia and Japan [11,12].
On MRI examination, ventricular enlargement and dilated cerebral and cerebellar sulci are common observations in dogs with NCL. These distinctive observations imply atrophy of the forebrain [6]. Similar changes have been observed in other lysosomal diseases, such as GM1 gangliosidosis and GM2 gangliosidosis in dogs [13,14]. The brain atrophy commonly progresses into a specific and severe change at the middle stage in NCL but as a secondary and mild change in the late-to-terminal stage in other lysosomal diseases. Therefore, brain atrophy may be helpful as an adjunct to the diagnosis of NCL.
NCL can be confirmed through detection of lipofuscin autofluorescence, H&E staining, PAS staining, and immunohistochemistry for LAMP-1. Despite the varying clinical course and ages of onset, all forms of NCL have unifying pathomorphological features. In this case, lipofuscin autofluorescence under ultraviolet light was observed. It is known that PAS is resistant to lipid solvents in the cytoplasm of most nerve cells and, to a lesser degree, in many other cell types [7]. Storage material consisting of lipid in the cytoplasm of neurons was identified in this case. LAMP-1 positivity in immunohistochemistry indicated alterations in this protein in the lysosome membrane. H&E staining, revealed inclusion bodies in the Purkinje cell cytoplasm, confirming that the cells had expanded and nuclei were compressed by the inclusion bodies. Additionally, the number of Purkinje cells had decreased. It was possible to confirm that the storage material stained with PAS in the cytoplasm. LAMP-1 was also detected in the cytoplasm.
Based on these results, the dog in this case report was diagnosed with NCL. However, the genotype could not be confirmed because no genetic testing was performed. Genetically, 14 types of abnormalities are classified, and among them, 8 types show neurological symptoms similar to human NCL [15]. In dogs, the causative mutation has been identified in many breeds, including the American Bulldog (CLN10), Border Collie (CLN5), English Setter (CLN8), Tibetan Terrier (CLN12), and Australian Shepherd mix (CLN8) [4]. Therefore, considering the Border Collie breed in this case, the cause of NCL was presumed to be a CLN5 mutation.
This is the first report to describe the MRI and histopathological features of NCL in a dog in Korea. NCL is a rare lysosomal storage disease in dogs that is relatively rare in veterinary medicine. The limitations of MRI should be recognized, and for definitive diagnosis, histological evaluation and gene sequencing are essential. Additionally, the mechanism by which NCL occurs is not clearly understood; therefore, further studies are required to elucidate this pathological mechanism.
Notes
The authors declare no conflict of interest.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2021R1A2C1012058) and the Basic Research Lab Program (2022R1A4A1025557) through the NRF of Korea, funded by the Ministry of Science and ICT.
