Primary copper-associated chronic hepatitis without copper metabolism domain containing 1 mutation in a Dalmatian: a case report
Article information
Abstract
A 12-year-old intact male Dalmatian dog presented hyporexia and vomiting for 1 week. Blood analysis revealed increased liver enzyme activity. Histopathological examination of the liver confirmed chronic hepatitis with fibrosis and necrosis. Copper staining revealed marked copper accumulation (2,770 ppm; normal range, 200 to 400 ppm), prominent in the centrilobular region, and compatible with copper-associated chronic hepatitis. However, copper metabolism domain containing 1 (COMMD1) mutation predisposing to copper accumulation in the liver tissue was not identified. The dog received medications but died 1 month after first visit. This is the first case of primary copper-associated hepatitis without COMMD1 mutation in a Dalmatian dog in South Korea.
The liver is an important organ for maintaining copper homeostasis, which stores copper and excretes excessive copper via the biliary system [1,2]. Excessive accumulation of copper in the hepatocytes can cause oxidative damage and inflammation, leading to copper-associated hepatitis [3–6]. Copper-associated hepatitis is diagnosed when significant copper accumulation is identified in the centrilobular areas (zone 3) on histological examination and copper concentrations over 1,000 ppm are confirmed in copper quantitation [7].
Copper-associated hepatitis can be classified into 2 broad categories, primary and secondary, depending on the underlying cause [4]. Primary copper-associated hepatitis is mainly related to genetic defects in copper metabolism and this type of disease has been reported in many breeds of dog, including Bedlington Terrier, Labrador Retriever, Doberman Pinscher, Dalmatian, West Highland White Terrier, and Skye Terrier [3–5]. In Bedlington Terrier, this disease was an inherited autosomal recessive disorder caused by a large deletion in exon 2 of the copper metabolism domain containing 1 (COMMD1) gene [1,6,8,9]. However, except for the Bedlington Terrier, the exact mechanisms of this disease are unknown in other breeds [3,10]. Recent reports of copper-associated hepatitis in dogs with no genetic predisposition, such as German Shepherds and Cocker Spaniels, suggest that environmental exposure to unknown factors may influence the pathogenesis of the disease [4].
A few cases of copper-associated hepatitis have been reported in Dalmatians in North America. Recently, copper-associated hepatitis has been reported in Dalmatians outside of North America. However, the patient was only 25 months old [3]. The present report describes the clinical features of primary copper-associated hepatitis diagnosed, based on histopathological findings and copper quantitation without COMMD1 mutation, in an elderly Dalmatian patient in South Korea.
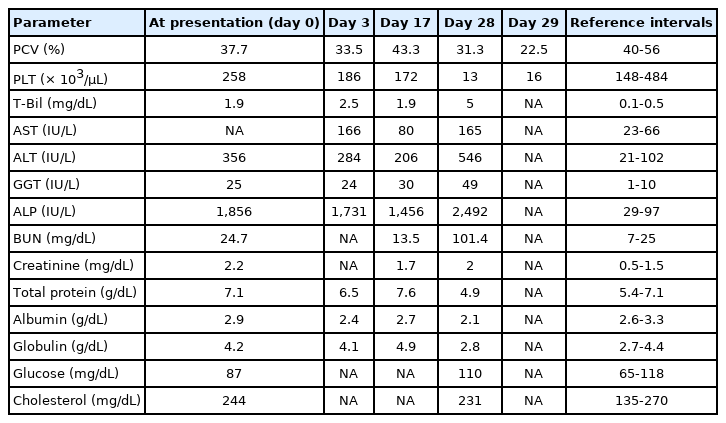
A 12-year-old intact male purebred Dalmatian dog weighing 18.35 kg presented hyporexia and vomiting for 1 week. The dog had no history of exposure to drugs or toxins. Jaundice of the sclera and skin were observed on physical examination upon admission. No abnormalities were observed in complete blood counts. Serum chemistry analysis revealed elevations in serum alanine aminotransferase (ALT), γ-glutamyl transferase (GGT), and alkaline phosphatase (ALP) activities and total bilirubin concentration (T-Bil) (Table 1). Abdominal radiography revealed that the liver was moderately small, but no other abnormal findings were observed. The liver was diffusely hyperechoic, coarse, and had an irregular contour and margin on ultrasonographic examination (Fig. 1).

Abdominal ultrasonographic images of the liver. (A) Diffusely coarse and mixed hypoechoic (black asterisk) and hyperechoic (white asterisk) echotexture of the liver parenchyma is observed. (B) A liver with round and irregular margin is identified (white arrow).
The patient was fed a commercial prescribing diet (Royal Canin hepatic diet; Royal Canin, France) containing 5 mg copper/kg dry matter. Furthermore, the dog received hepatoprotectants, including ursodeoxycholic acid (Ursa; Daewoong Pharm., Korea) 10 mg/kg twice daily, vitamin E (Grandpherol; Yuhan Co., Korea) 400 IU/dog once daily, and s-adenosylmethionine (Zentonil advanced; Vetoquinol, France) 20 mg/kg once daily to prevent further liver damage. After administration of the hepatic protectant, the serum activities of aspartate aminotransferase (AST), ALT, GGT, and ALP decreased, but hyporexia persisted (Table 1). Ultrasound-guided liver biopsy was conducted under general anesthesia to diagnose the underlying liver parenchymal disease 4 days after the first visit.
Histopathological findings of the liver biopsy samples exhibited many inflammatory infiltrates of neutrophils, Kupffer cells, small mature lymphocytes, and plasma cells. The inflammatory infiltrates were primarily composed of neutrophils and lymphocytes. Furthermore, moderate fibrosis, biliary hyperplasia, and multifocal necrosis of hepatocellular cells confirmed chronic hepatitis, mainly in the centrilobular region (zone 3) (Fig. 2). Subsequently, rhodanine copper staining was performed and it revealed marked copper accumulation in the liver, which involved all regions, but was most prominent in the centrilobular region (zone 3) (Fig. 2). The scale of copper accumulation was grade 5, ranging from 0 (no copper granules) to 5 (panlobular presence of copper granules) [11]. Quantitative analysis of copper revealed 2,770 ppm (normal range, 200 to 400 ppm per dry weight of liver tissue), indicating excessive copper accumulation [6].

Histological section of the liver. (A, B) Necrotic hepatocytes (black arrows) and inflammatory cell infiltration (asterisks). Vacuolation and proliferated bile ducts is also observed. H&E, scale bar: 100 μm. (C) Moderate fibrosis are identified by blue color (asterisk). Trichrome stain, scale bar: 200 μm. (D) Copper granules are stained red-brown (black arrow). Rhodanine stain, scale bar: 100 μm.
Genetic testing using polymerase chain reaction was performed to identify a mutation in the COMMD1 gene, which was related to the copper-associated hepatitis in the Bedlington Terrier. However, this mutation was not observed in the present case. Although the cause of copper accumulation in this case could not be identified using genetic information, copper-associated chronic hepatitis was diagnosed and confirmed based on the histological findings and copper quantitation.
The dog was administered with 10 mg/kg D-penicillamine (Artamin; Ildong Pharm., Korea) twice daily as a copper-chelating agent. After 11 days of treatment with D-penicillamine, serum AST and ALT levels decreased (Table 1), and no adverse effects were identified. The dose of D-penicillamine was increased to 15 mg/kg twice daily. However, the dog showed lethargy, anorexia, and abdominal distension 11 days after the dose escalation of D-penicillamine. Additionally, pale mucous membranes and purpura were observed throughout the body. Ascites was detected by abdominal ultrasonography, and a total of 1,950 ml was drained from the abdominal cavity. Ascites of the dog was a pure transudate (total nuclear cell counts = 80 cells/µL, total protein concentration = 0.3 g/dL, and albumin concentration = 0.2 g/dL). Serum biochemical analysis revealed hypoalbuminemia, elevated liver enzyme activities, severely increased T-Bil concentration, and azotemia (Table 1). The dog died the next day, approximately one month after the first visit. Necropsy was not conducted owing to refusal of the owner.
Copper accumulation in the liver can result from primary copper accumulation due to a genetic defect in the hepatic copper metabolism, or secondary copper accumulation due to changes in the biliary excretion of copper, or increased copper uptake [3,6,12]. Primary copper accumulation is commonly observed in Bedlington Terriers, which have a genetic disorder in copper metabolism because of mutations in the COMMD1 gene required for copper excretion [1,3,7,12]. Secondary copper accumulation is a result of liver diseases such as cholestasis, leading to impaired excretion of copper [3,6]. The region of copper accumulation can help to distinguish between primary and secondary copper accumulations [12]. The primary copper accumulation is prominent in the centrilobular areas (zone 3), whereas the secondary copper accumulation is mainly limited to the periportal regions (zone 1) [3,6,12]. Moreover, copper concentrations above 1,000 ppm in the liver are consistent with primary accumulation, whereas copper concentrations range from 400 to 1,000 ppm in secondary copper accumulation [12]. The precise cause of copper accumulation in Dalmatians has not yet been reported. However, histological evidence of copper accumulation in this case was prominent in the centrilobular areas (zone 3) and 2,770 ppm copper concentrations were confirmed, indicating a high probability of primary copper accumulation [3,6].
The mean survival time of dogs with chronic hepatitis is approximately 561 days, and it has been reported that many dogs die from liver disease-related causes [7]. However, this patient died approximately one month after the first visit. The exact cause of death was unknown as necropsy was not performed. Ascites, hyperbilirubinemia, hypoalbuminemia, purpura throughout the body, and gastrointestinal bleeding were observed in the patient before death, all of which indicate poor prognosis in hepatic disease [7]. Besides, liver fibrosis identified by histological findings in this case was also a poor prognostic factor [7]. In addition, the presence of a pure transudative ascites with serum albumin levels greater than 1.6 g/dL can lead to the diagnosis of portal hypertension [13]. Therefore, these factors may have affected the shortening of the patients survival period. Not only the progression of chronic hepatitis but also the possibility of side effects such as anemia and thrombocytopenia due to increased D-penicillamine dose before death were considered. The cause of death is most likely due to the rapid progression of liver failure, but adverse effects of D-penicillamine, gastrointestinal hemorrhage due to coagulopathy, and unexplained causes may also be suggested.
In this case, genetic testing was performed to identify an association with the genetic disorder; however, the COMMD1 gene related to copper-associated hepatitis in the Bedlington Terrier was not confirmed. Many proteins are involved in copper metabolism, hence, the possibility of other gene mutations could not be ruled out [1,5,6]. Mutations in the ATP7B gene, which allows copper excretion into bile, have been reported to be associated with copper accumulation in the liver of Labrador Retrievers [1,2,7,10]. However, this report has a limitation that the test for ATP7B gene mutation was not performed in this case.
Bedlington terriers with an identified genetic cause tend to start accumulating copper between 6 and 12 months of age, without other histological signs [1]. However, Dalmatians tend to be diagnosed with copper-associated hepatitis at a later age than Bedlington Terriers [14]. This patient was 12-year-old at the time of diagnosis, and the mean age of other reported Dalmatians with copper-associated hepatitis was 6 years [14]. This can be considered not only the possibility of genetic factors in purebred Dalmatians but also the possibility of other cofactors involved in the expression of gene mutations or copper accumulation.
The precise cause of copper-associated hepatitis in Dalmatians remains to be elucidated. Therefore, further studies should focus on identifying the genetic basis for copper accumulation in Dalmatians, and additional research is needed on epidemiological factors, such as environmental exposure, that may influence the expression of genetic mutations.
There have been reports of copper-associated hepatitis in Dalmatians, but most of these cases occurred in North America, with only a small number of cases. One such case was recently reported in Japan [3,14,15]. To our knowledge, this is the first case of copper-associated chronic hepatitis in a Dalmatian dog in South Korea. Clinicians should be aware that copper-associated hepatitis should be considered as a differential diagnosis for primary liver disease in Dalmatians, and copper quantification should be recommended.
Notes
The authors declare no conflict of interest.
Acknowledgements
The authors would like to thank the owners of the dog included in this report. This work was supported by the Global Research and Development Center (GRDC) Program through the National Research Foundation (NRF) of Korea funded by the Ministry of Science and ICT (2017K1A4A3014959) and Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through Agriculture, Food and Rural Affairs Convergence Technologies Program for Educating Creative Global Leader, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (grant number: 320005–4).