Differential cytotoxic effects of fenbendazole on mouse lymphoma EL-4 cells and spleen cells
Article information
Abstract
Fenbendazole (FBZ) is a benzimidazole anthelmintic widely used to treat parasitic infections. The anticancer effect of FBZ has been recently highlighted leading to its consideration as a potential anticancer agent. Although previous studies have demonstrated the effect of FBZ on cancer cells, there is a paucity of studies on the effect of FBZ on lymphoma cells and normal immune cells. Herein, we investigated the effects of FBZ on a mouse lymphoma cell line, EL-4 cells, and spleen cells, using vincristine as a positive control. The cellular metabolic activity of EL-4 cells was decreased by FBZ, but that of the spleen cells was not decreased. Moreover, FBZ reduced the mitochondrial membrane potential and induced reactive oxygen species production in EL-4 cells, but not in spleen cells. FBZ induced G2/M phase arrest and increased the sub G0/G1 phase ratio, indicating apoptosis. Furthermore, compared to the control cells, the reactivity of spleen cells pretreated with FBZ to lipopolysaccharide was maintained. In summary, FBZ is cytotoxic to EL-4 cells, but not to spleen cells. This study provides experimental evidence that FBZ exerts an anticancer effect, and less cytotoxic effects and functional damage to normal spleen cells.
Introduction
Fenbendazole (FBZ) is a benzimidazole anthelmintic commonly used to treat parasitic infections, including helminth infections, in livestock, companion animals, and humans [1,2]. FBZ exerts an antiparasitic effect by binding to tubulin and disrupting tubulin equilibrium. The binding of FBZ to the tubulin of parasites exerts a stronger inhibitory effect than in mammals, causing immobilization and death of the parasites [3]. Recently, owing to its selective binding to microtubules, FBZ has been widely studied as a repurposed drug for anticancer therapy [4,5].
Some anticancer drugs, including vincristine (VC) and paclitaxel, exert antineoplastic effects by interfering with microtubule formation and depolymerization [6,7], which is similar to the mechanism of action of benzimidazole anthelmintics. As these anticancer drugs have toxic adverse effects on normal cells and cause immunosuppression, considerable caution is needed prior to their use. In particular, repeated cycles of chemotherapy affect the function of lymphocytes and decrease T cell-mediated immune responses [8,9]. Therefore, before repurposing FBZ as an anticancer drug, it is important to evaluate its cytotoxic effects on cancer cells while minimizing its effect on normal immune cells.
Thus, in this study, we investigated the effect of FBZ on a mouse lymphoma cell line, EL-4, and normal spleen cells. We determined the metabolic activity, apoptosis, and mitochondrial membrane potential (MMP) of both EL-4 and normal spleen cells following FBZ treatment, and performed cell cycle analyses in comparison with VC treatment, which acts as a positive control with a similar mechanism of action as FBZ.
Materials and Methods
Animals and reagents
C57BL/6 and BALB/c mice were purchased from Samtaco Bio Korea and housed in the animal facility at Jeju National University. In this study, 7 to 12-week-old mice were used and all animal experiments in this study were performed in accordance with the Institutional Guidelines for Animal Use and Care of Jeju National University (No: 2021-0047). FBZ was purchased from Sigma-Aldrich (USA) and dissolved in dimethyl sulfoxide (Sigma-Aldrich). VC was purchased from REYON Pharmaceutical Co. (Korea) and was used as a positive control for anticancer drugs. Lipopolysaccharide (LPS) purified from Escherichia coli O26:B6 was purchased from Sigma-Aldrich and dissolved in sterile phosphate-buffered saline (PBS).
Cell lines
The EL4 (mouse lymphoma cell line) was purchased from Korean Cell Line Bank (Korea). The cells were cultured in RPMI 1640 medium containing 10% fetal bovine serum, 100 U/mL penicillin-streptomycin, and 2 mM L-glutamine.
Preparation of spleen cells
The spleen was harvested and physically crushed to obtain cells. After hemolysis and removal of red blood cells using ammonium chloride potassium lysis buffer (Thermo Fisher Scientific, USA), spleen cells were filtered through a 70 µm cell strainer. RPMI 1640 medium containing 10% fetal bovine serum, L-glutamine, and penicillin-streptomycin was added and the cells were ready to be cultured.
Measurement of the cellular metabolic activity
To measure the metabolic activity of the cells, EL-4 and spleen cells were treated with FBZ or VC and cultured in 96-well plates for 3 days. EL-4 and spleen cells were cultured at concentrations of 1 × 105 and 2 × 106 cells/mL, respectively. A 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenylltetrazolium bromide (MTT) solution was added at a concentration of 0.5 mg/mL and incubated at 37°C in 5% CO2 for 4 hours. A 10% sodium dodecyl sulfate solution was added to dissolve the formazan product (crystal violet) produced by the cells and reacted for 2 hours. Optical density was measured at 570 nm using a microplate reader (Multiskan FC; Thermo Fisher Scientific).
Flow cytometry analysis
Apoptosis, MMP, reactive oxygen species (ROS), and cell cycle analysis were performed using flow cytometry. The EL-4 and spleen cells were treated with FBZ or VC in 24-well plates and incubated for the required number of days, depending on the experiment. For VC, the IC50 was used as the representative concentration (25 ng/mL). EL-4 and spleen cells were cultured at concentrations of 1 × 105 and 1 × 106 cells/mL, respectively. To determine apoptosis, the cells were stained with annexin V-fluorescein isothiocyanate (FITC) (Thermo Fisher Scientific) and washed with annexin V-binding buffer. Subsequently, 1.5 µg/mL propidium iodide (PI; Sigma-Aldrich) were added at room temperature for 10 minutes in the dark. To measure MMP, cells were incubated for 48 hours and treated with 0.1 µg/mL rhodamine 123 (Sigma-Aldrich) for 30 minutes in the dark. The treated cells were washed twice with the fluorescence-activated cell sorting staining solution (FSS). For ROS detection, cells were treated for 72 hours, washed with pre-warmed PBS, and stained with 10 μM 2',7'–dichlorofluorescin diacetate (DCFDA, Sigma-Aldrich). After 1 hour incubation at 37°C, the stained cells were washed with FSS twice. Then, 2',7'-DCF, an oxidized form of DCFDA produced by ROS was measured. For cell cycle analysis, DNA content was evaluated by PI staining. Following treatment with FBZ or VC, the treated cells were washed with PBS and fixed with 70% cold ethanol for 20 minutes at -20℃. After the fixative was removed, the cells were stained with PI solution at a final concentration of 50 μg/mL, with 0.2 mg/mL RNase. All stained cells were analyzed using flow cytometry (CytoFLEX LX; Beckman Coulter, USA) and CytExpert software (Beckman Coulter).
Reactivity of FBZ-treated spleen cells to LPS
To investigate whether FBZ-treated spleen cells can respond to LPS stimulation, spleen cells were pretreated with FBZ at a concentration of 2 × 106 cells/mL. After 24 hours, the cells were harvested and washed with culture media, and the 2 × 106 cells/mL viable cells were treated with 1 µg/mL LPS. The cells were incubated for another 48 hours, and the metabolic activity was measured using the MTT assay.
Statistical analysis
Statistical analyses were performed using Prism 9 (GraphPad Software, USA). Data are expressed as the mean ± standard deviation. All experiments were performed at least three times. Statistical significance was analyzed using ANOVA and an appropriate multiple comparison test.
Results
The effect of FBZ and VC on EL-4 cells and spleen cells
Using the MTT assay, the metabolic activity of EL-4 cells and spleen cells was evaluated after treatment with FBZ or VC (Fig. 1). The metabolic activity of EL-4 decreased significantly at all concentrations, and significantly dropped at concentrations greater than 0.5 µM (p < 0.0001). The metabolic activity significantly decreased at some concentrations in the spleen cells, but was less than the effect observed in EL-4 cells.
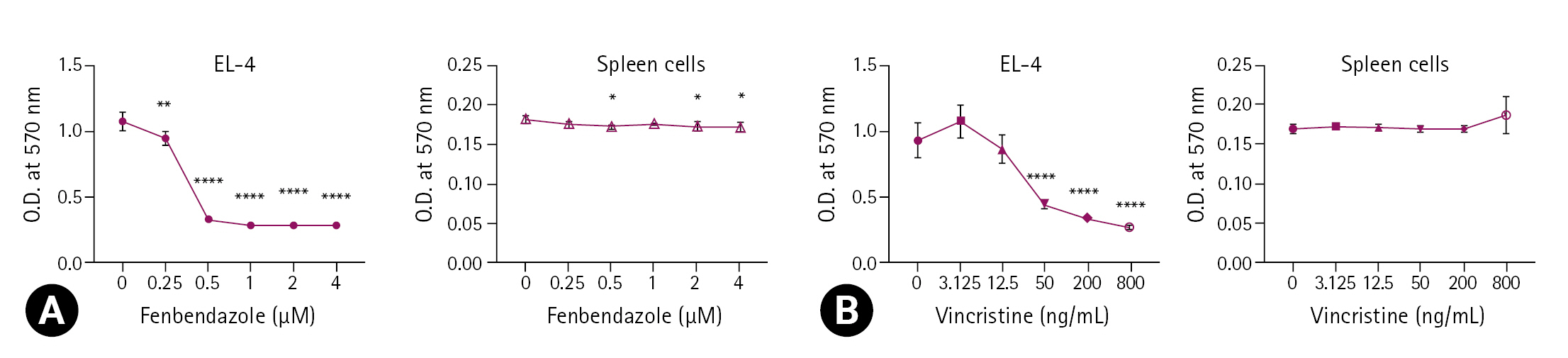
The effect of fenbendazole and vincristine on the metabolic activity of EL-4 cells and spleen cells. EL-4 cells were cultured in 96-well culture plate at a concentration of 1 × 105 cells/mL and 2 × 106 cells/mL, respectively and treated with 0 to 4 μM fenbendazole (A) and 0 to 800 ng/mL vincristine (B), for 3 days. MTT assay was performed as described in “Materials and Methods”. The optical density (O.D.) was measured at 570 nm using a microplate reader. The results are presented as the mean ± standard deviation, and the statistical significance was determined by one-way ANOVA followed by Dunnett’s multiple comparisons test. *p < 0.05, **p < 0.01, and ****p < 0.0001 compared to the control.
FBZ increases the cell death of EL-4 cells
Treatment of EL-4 cells with FBZ revealed a decrease in the percentage of live cells in a concentration-dependent manner (Fig. 2). We observed a 52% reduction in the ratio of viable cells following treatment of EL-4 cells with 1 µM FBZ, compared with the controls (0 µM FBZ). On the other hand, treatment of spleen cells with 1 µM FBZ revealed a 9% reduction in the ratio of viable cells compared to the controls. This result confirmed that FBZ markedly induced the death of EL-4 cells compared to spleen cells.
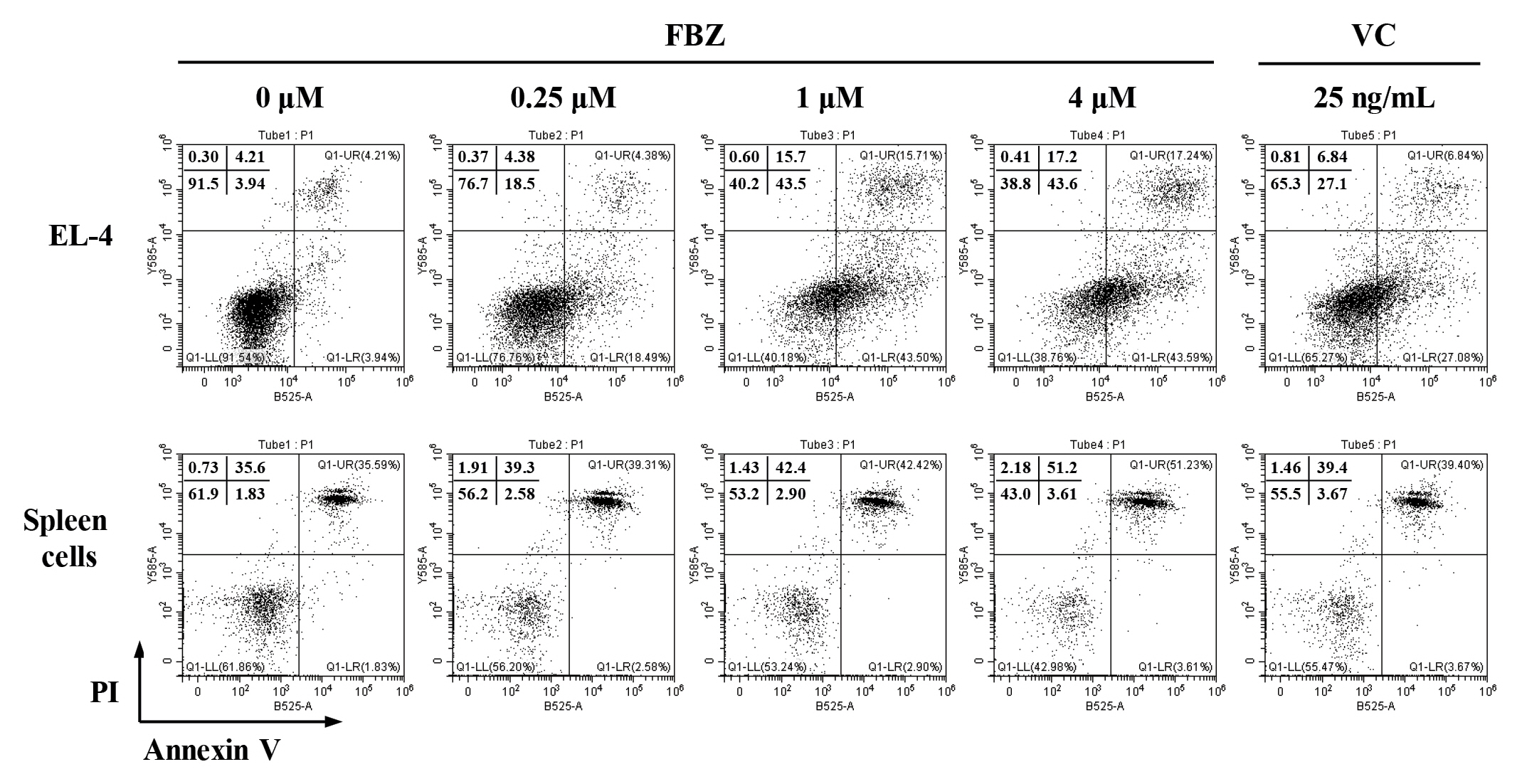
Fenbendazole (FBZ) increases the cell death of EL-4 cells. The cell death of EL-4 cells and spleen cells were analyzed by staining with Annexin V-fluorescein isothiocyanate (V-FITC) and propidium iodide (PI). The quadrants represent the percentage of the cells: viable cells (lower left), early apoptotic cells (lower right), late apoptotic cells (upper right), and necrotic cells (upper left). VC, vincristine.
FBZ induces ROS production in EL-4 cells
After excluding the estimated cell debris, ROS production in cells was measured using flow cytometry. In EL-4 cells, FBZ increased the mean fluorescence intensity (MFI) of DCFDA in a concentration-dependent manner (Fig. 3A). In particular, a 48% and 53% increase in the rate of ROS production at 1 µM and 4 µM FBZ compared to the control (0 µM) group was observed. In spleen cells, the tendency of ROS production was completely different; the MFI in spleen cells treated with DCFDA was not significantly different at all concentrations compared to the controls. VC (25 ng/mL) showed a similar trend of ROS production as FBZ in both cell types.
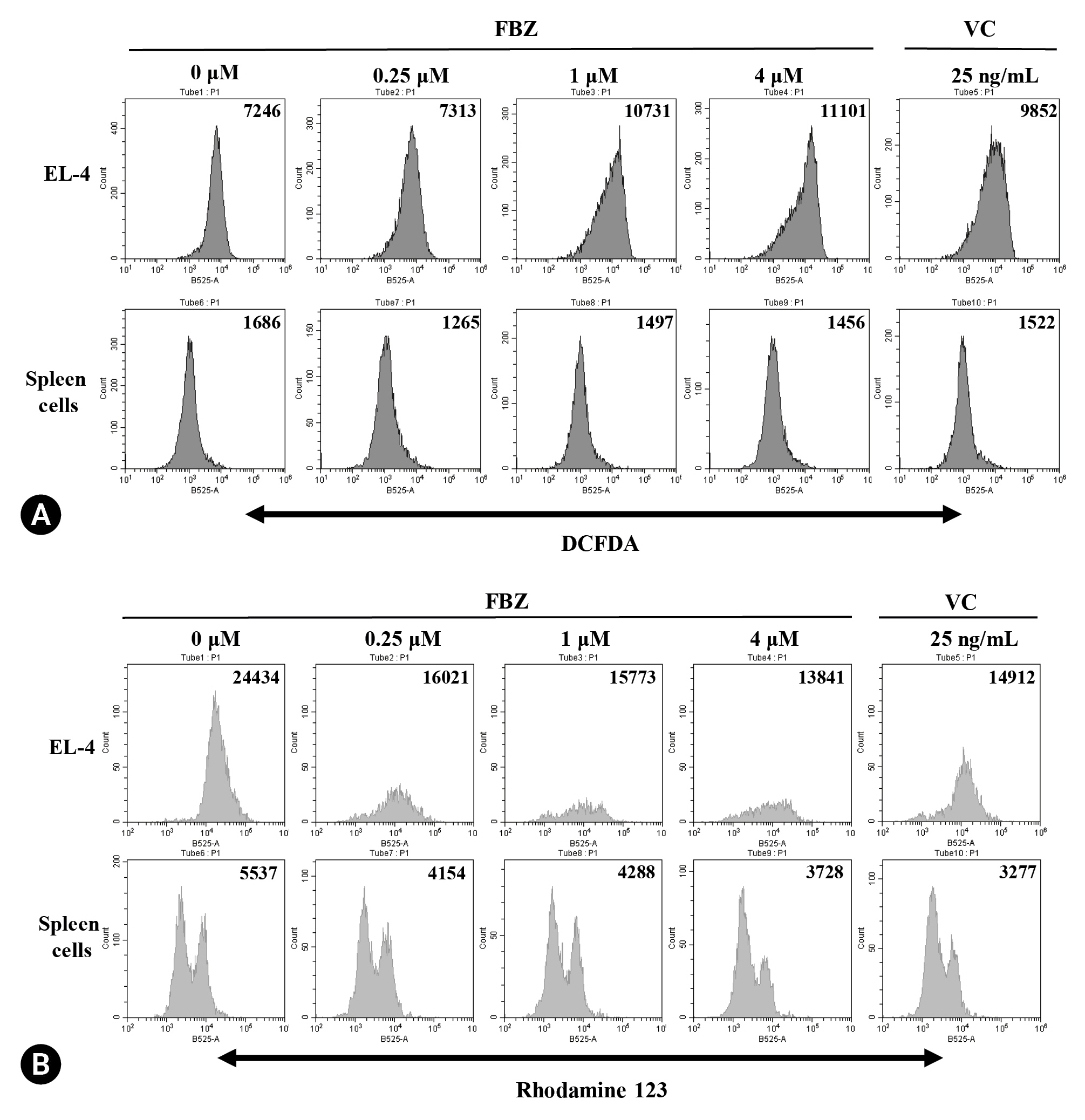
Effect of fenbendazole (FBZ) on reactive oxygen species (ROS) production and MMP. EL-4 cells and spleen cells were treated with FBZ and vincristine (VC) for 72 hours and 48 hours to measure the ROS production by dichlorofluorescin diacetate (DCFDA) staining (A) and MMP by Rhodamine 123 staining (B). Stained cells were analyzed by flow cytometry and the numbers in the histograms indicate the mean fluorescence intensity.
Influence of FBZ on mitochondrial membrane potential
To investigate whether FBZ influences mitochondrial integrity of cells, we treated EL-4 cells with FBZ and stained them with rhodamine 123 solution. The MFI of rhodamine 123 was decreased by FBZ in a concentration-dependent manner in both EL-4 cells and spleen cells (Fig. 3B). At the final concentration of FBZ (4 µM), MMP was decreased by 43% and 33% in EL-4 cells and spleen cells, respectively. When treated with 25 ng/mL VC, the MMP decreased by 39% and 41% in EL-4 cells and spleen cells, respectively.
FBZ induces G2/M phase arrest in EL-4 cells
To determine whether FBZ affects the cell cycle, we treated EL-4 cells with FBZ and stained with PI solution. As shown in Fig. 4, the fraction of EL-4 cells in the G2/M phase increased at concentrations of 1 and 4 µM. The fraction of cells in the S phase was decreased, which was accompanied by a marked increase in the fraction of cells in the sub G0/G1 phase. In contrast, in spleen cells, each fraction of the cell cycle was not notably altered by FBZ treatment. This result confirmed that FBZ causes a distinct change in the G2/M phase of the cell cycle of EL-4 cells.
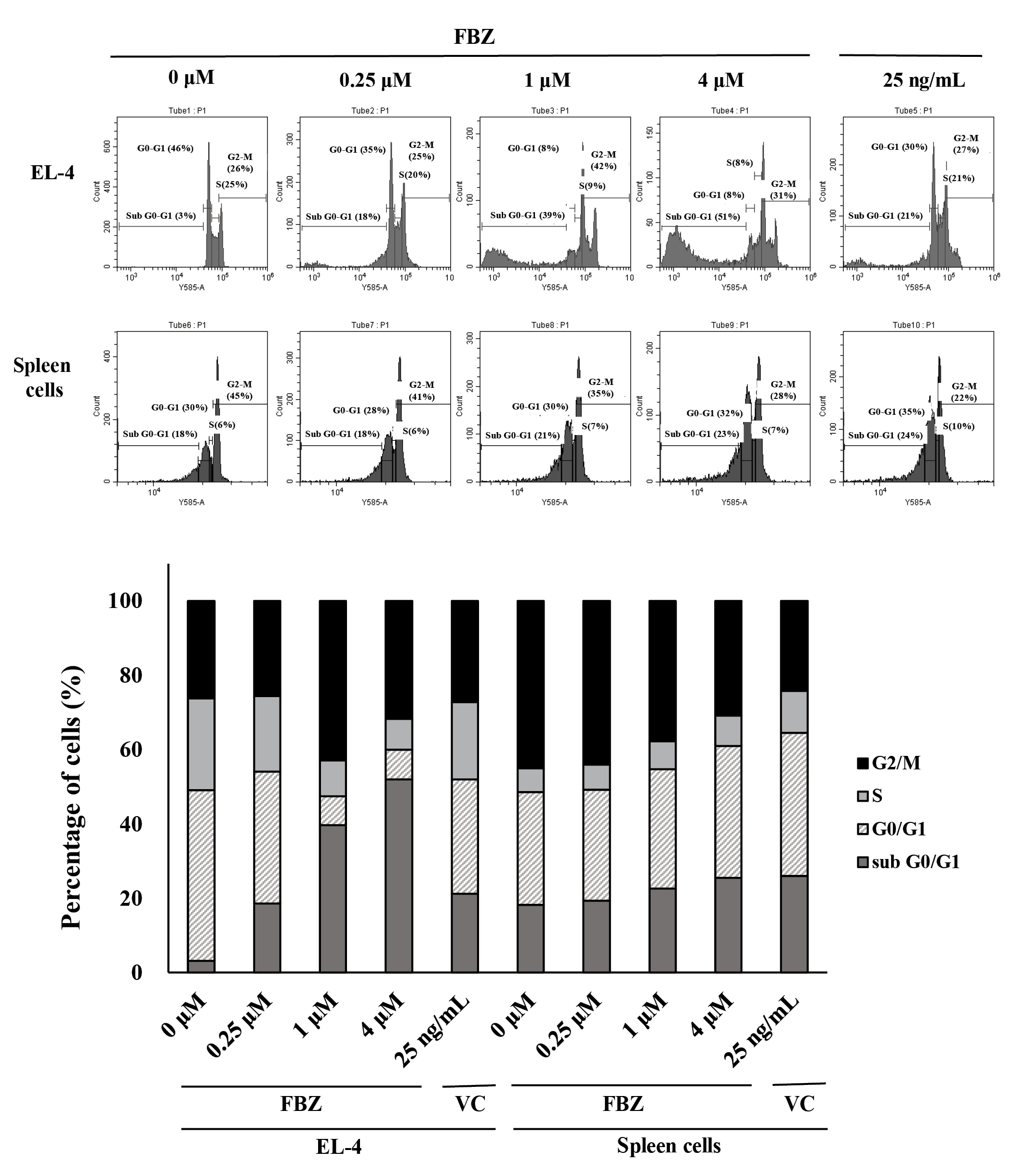
Fenbendazole (FBZ) induces G2/M phase arrest in EL-4 cells. Cells were treated with FBZ or vincristine (VC) for 3 days and stained with propidium iodide. Cell cycle differences of EL-4 cells and spleen cells were assessed by flow cytometry. The percentage of EL-4 cells and spleen cells in each phase is shown.
FBZ-pretreated spleen cells normally respond to LPS
We investigated whether spleen cells treated with FBZ could maintain their activation status. First, spleen cells were pretreated with FBZ for 24 hours, and then viable cells were stimulated with LPS for another 48 hours (Fig. 5). The cells were significantly activated at all FBZ concentrations. Although the number of viable spleen cells was decreased by 21% at 1 µM FBZ (data not shown) compared to control (0 µM FBZ), the activation ability of the viable spleen cells to LPS stimulation did not decrease. These data reveal that FBZ had a minimal effect on the responding ability of viable spleen cells to LPS.
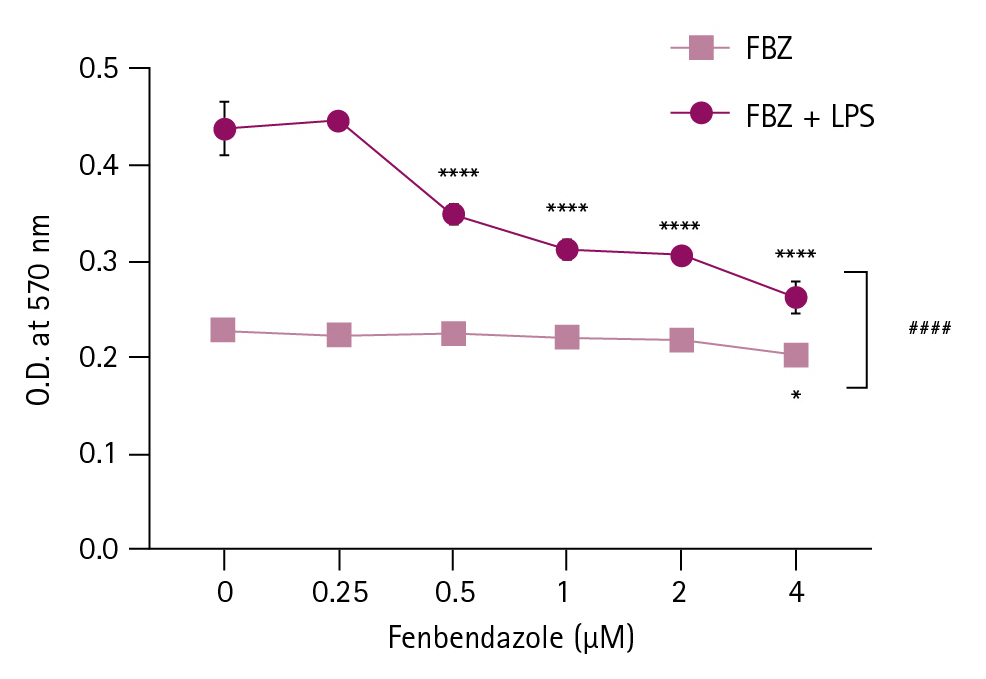
Reactivity of fenbendazole (FBZ)-pretreated spleen cells to lipopolysaccharide (LPS). Spleen cells were pretreated with FBZ for 24 hours. Viable cells were counted and setup in 96-well culture plates in the presence of LPS for another 48 hours. Then, the cellular metabolic activity of spleen cells was measured at 570 nm using a microplate reader. The results are presented as mean ± standard deviation, and the statistical significance was performed by two-way ANOVA followed by Šídák’s multiple comparisons test. *p < 0.05, and ****p < 0.0001 compared to control cells (FBZ 0 µM) and ####p < 0.0001 between the groups with and without LPS treatment at the same FBZ concentration. O.D., optical density.
Discussion
The potential of FBZ, a benzimidazole anthelmintic, as an anticancer agent is receiving increasing attention [4,5]. The purpose of this study was to investigate the cytotoxic effects of FBZ on cells of the spleen, which is a major reservoir of immune cells. We compared the cytotoxic effects of FBZ on a lymphoma cell line, EL-4 cells, and spleen cells using various assays.
Some widely used anticancer drugs, such as VC and Paclitaxel, target microtubules and disturb cell division, among the diverse anticancer mechanisms [6,7]. FBZ also binds to β-tubulin within the microtubules and inhibits its formation; and possesses a similar mechanism of action to that of VC [10,11]. Both FBZ and VC showed similar cellular metabolic activity in EL-4 and spleen cells (Fig. 1). FBZ and VC induced a drastic decline in the metabolic activity of EL-4 cells, but did not affect that of spleen cells in the same concentration range.
Based on the MTT assay (Fig. 1), we determined the IC50 of FBZ and VC on EL-4 cells as 0.32 µM (= 95.8 µg/mL) and 23.58 ng/mL, respectively. We compared the effect of FBZ and VC on both EL-4 and spleen cells at their approximate IC50 for EL-4 cells; 0.25 µM and 25 ng/mL, respectively. The annexin V/PI assay demonstrated that the viable cells decreased by 52% in EL-4 cells and 9% in spleen cells at 1 µM FBZ (Fig. 2). In EL-4 cells, 25 ng/mL VC generated more ROS and reduced MMP, similar to 1 µM FBZ than 0.25 µM FBZ. In spleen cells, FBZ and VC had no influence on ROS production, but MMP decreased to a comparable level at 4 µM FBZ and 25 ng/mL VC (Fig. 3). The cell cycle of the EL-4 cells was markedly altered by FBZ treatment (Fig. 4). The fraction of cells in the G2/M phase increased, and that in the S phase decreased. In contrast, there were no notable changes in spleen cells. These data demonstrated that FBZ induced G2/M phase arrest and increased the sub G0/G1 phase ratio, indicating apoptosis in EL-4 cells [12]. Overall, FBZ had strong cytotoxic effects on EL-4 cells compared to spleen cells.
To investigate the activation capability of FBZ-pretreated spleen cells, we used LPS as a lymphocyte-stimulating agent (Fig. 5). At 1 µM FBZ, the viability of spleen cells decreased and the reactivity of FBZ-pretreated spleen cells to LPS was maintained. In this study, although the viability of spleen cells was slightly decreased by FBZ, there were few changes in metabolic activity, ROS production, and cell cycle, whereas the activation ability of FBZ-treated spleen cells was maintained.
In this study, we confirmed that the anticancer effect of FBZ on a mouse lymphoma cell line, EL-4, was similar to that of VC, which is a widely used chemotherapeutic agent for lymphoma. In addition, FBZ has minimal cytotoxicity on spleen cells, which are primary immune cells. Taken together, this study demonstrates the possibility of repurposing FBZ as an anticancer drug and provides valuable information to broaden the use of FBZ in clinical and basic research.
Notes
The authors declare no conflict of interest.
Acknowledgements
This research was supported by the 2022 scientific promotion program funded by Jeju National University.
