2. Zhou Q, Yang SH, Ding CH, He XC, Xie YH, Hildebrandt TB, Mitalipov SM, Tang XH, Wolf DP, Ji WZ. A comparative approach to somatic cell nuclear transfer in the rhesus monkey. Hum Reprod 2006;21:2564-2571.


3. Malin K, Witkowska-Piłaszewicz O, Papis K. The many problems of somatic cell nuclear transfer in reproductive cloning of mammals. Theriogenology 2022;189:246-254.


6. Dinnyes A, Tian XC, Yang X. Epigenetic regulation of foetal development in nuclear transfer animal models. Reprod Domest Anim 2008;43 Suppl 2:302-309.


9. Boediono A, Suzuki T, Li LY, Godke RA. Offspring born from chimeras reconstructed from parthenogenetic and
in vitro fertilized bovine embryos. Mol Reprod Dev 1999;53:159-170.


11. Hiriart MI, Bevacqua RJ, Canel NG, Fernández-Martín R, Salamone DF. Production of chimeric embryos by aggregation of bovine egfp eight-cell stage blastomeres with two-cell fused and asynchronic embryos. Theriogenology 2013;80:357-364.


13. Lee SG, Park CH, Choi DH, Kim HS, Ka HH, Lee CK. In vitro development and cell allocation of porcine blastocysts derived by aggregation of in vitro fertilized embryos. Mol Reprod Dev 2007;74:1436-1445.


14. Terashita Y, Sugimura S, Kudo Y, Amano R, Hiradate Y, Sato E. Improving the quality of miniature pig somatic cell nuclear transfer blastocysts: aggregation of SCNT embryos at the four-cell stage. Reprod Domest Anim 2011;46:189-196.


16. Ribeiro ES, Gerger RP, Ohlweiler LU, Ortigari I, Mezzalira JC, Forell F, Bertolini LR, Rodrigues JL, Ambrósio CE, Miglino MA, Mezzalira A, Bertolini M. Developmental potential of bovine hand-made clone embryos reconstructed by aggregation or fusion with distinct cytoplasmic volumes. Cloning Stem Cells 2009;11:377-386.


17. Savy V, Alberio V, Vans Landschoot G, Moro LN, Olea FD, Rodríguez-Álvarez L, Salamone DF. Effect of embryo aggregation on in vitro development of adipose-derived mesenchymal stem cell-derived bovine clones. Cell Reprogram 2021;23:277-289.


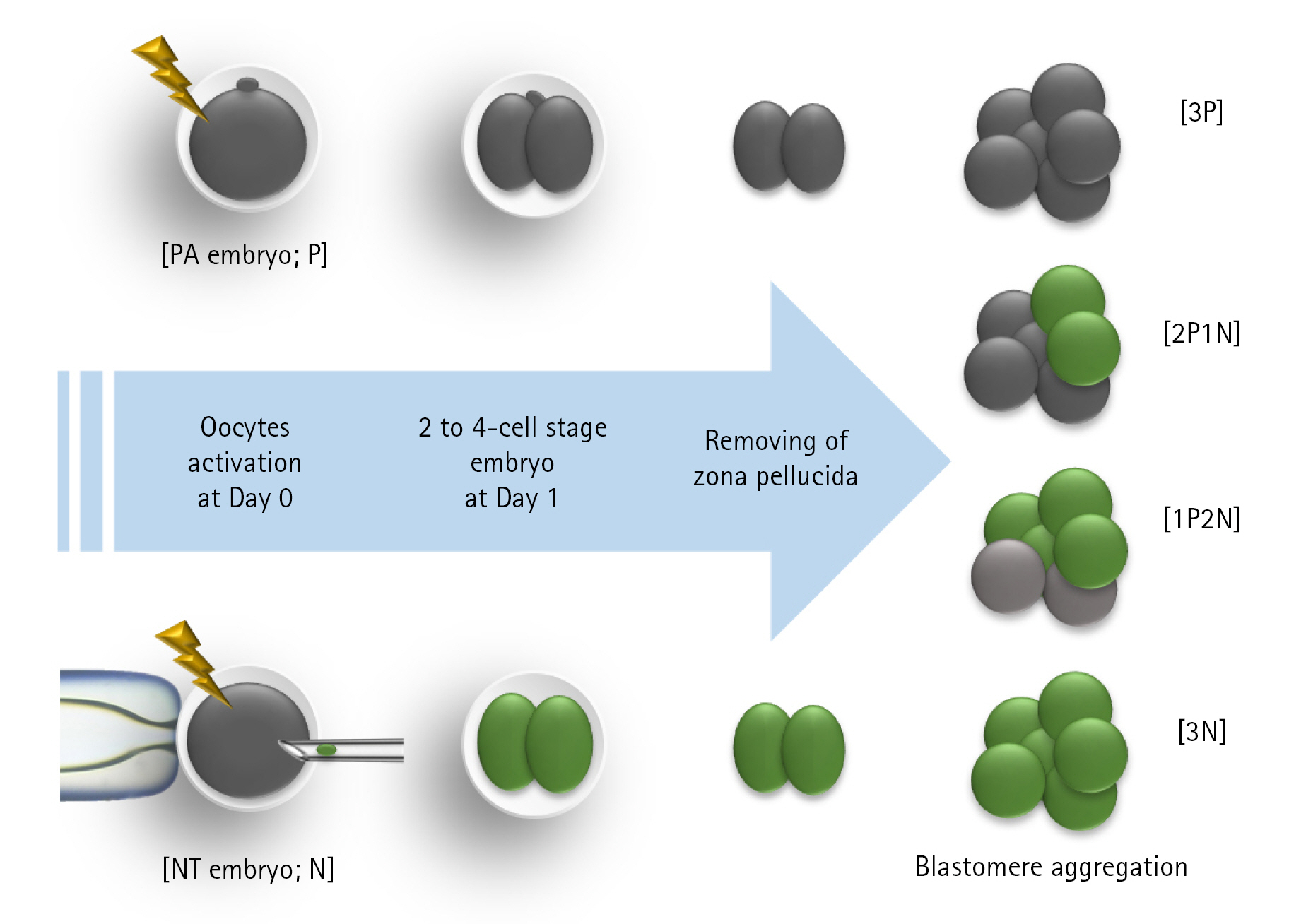
19. Li R, Liu Y, Pedersen HS, Kragh PM, Callesen H. Development and quality of porcine parthenogenetically activated embryos after removal of zona pellucida. Theriogenology 2013;80:58-64.


23. Naito K, Toyoda Y, Yanagimachi R. Production of normal mice from oocytes fertilized and developed without zonae pellucidae. Hum Reprod 1992;7:281-285.


24. Oback B, Wiersema AT, Gaynor P, Laible G, Tucker FC, Oliver JE, Miller AL, Troskie HE, Wilson KL, Forsyth JT, Berg MC, Cockrem K, McMillan V, Tervit HR, Wells DN. Cloned cattle derived from a novel zona-free embryo reconstruction system. Cloning Stem Cells 2003;5:3-12.


25. Sampaío MA, Geber S. Births after transfer of zona-free blastocysts in oocyte donation cycles. J Assist Reprod Genet 2001;18:156-159.


26. Mansour RT, Rhodes CA, Aboulghar MA, Serour GI, Kamal A. Transfer of zona-free embryos improves outcome in poor prognosis patients: a prospective randomized controlled study. Hum Reprod 2000;15:1061-1064.


27. Nichols J, Gardner RL. Effect of damage to the zona pellucida on development of preimplantation embryos in the mouse. Hum Reprod 1989;4:180-187.


28. Simmet K, Reichenbach M, Reichenbach HD, Wolf E. Phytohemagglutinin facilitates the aggregation of blastomere pairs from day 5 donor embryos with day 4 host embryos for chimeric bovine embryo multiplication. Theriogenology 2015;84:1603-1610.


29. Jeong PS, Yoon SB, Lee MH, Son HC, Lee HY, Lee S, Koo BS, Jeong KJ, Lee JH, Jin YB, Song BS, Kim JS, Kim SU, Koo DB, Sim BW. Embryo aggregation regulates in vitro stress conditions to promote developmental competence in pigs. PeerJ 2019;7:e8143.



31. Ji H, Long C, Feng C, Shi N, Jiang Y, Zeng G, Li X, Wu J, Lu L, Lu S, Pan D. Generation of chimeric minipigs by aggregating 4- to 8-cell-stage blastomeres from somatic cell nuclear transfer with the tracing of enhanced green fluorescent protein. Xenotransplantation 2017;24:e12300.

32. Tagawa M, Matoba S, Narita M, Saito N, Nagai T, Imai K. Production of monozygotic twin calves using the blastomere separation technique and Well of the Well culture system. Theriogenology 2008;69:574-582.


34. Duque Rodriguez M, Gambini A, Ratner LD, Sestelo AJ, Briski O, Gutnisky C, Rulli SB, Fernández Martin R, Cetica P, Salamone DF. Aggregation of Leopardus geoffroyi hybrid embryos with domestic cat tetraploid blastomeres. Reproduction 2021;161:539-548.


36. Paldi A, Nagy A, Markkula M, Barna I, Dezso L. Postnatal development of parthenogenetic in equilibrium with fertilized mouse aggregation chimeras. Development 1989;105:115-118.















 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



