Rabies neutralizing antibody titers in Korean dogs and cats intended for overseas travel
Article information
Abstract
Animals imported from abroad are a cause of rabies outbreaks in many countries. Therefore, rabies serology testing for dogs and cats traveling abroad is an important measure to reduce the incidence of rabies. Rabies virus antibodies were measured in sera collected from 2,367 dogs and 894 cats between 2017 and 2021. A serum sample with a value of 0.5 IU/mL or higher was considered a pass. The overall pass rates for rabies virus were 96.4% in dogs and 98.4% in cats. The mean rabies virus neutralization assay titers were higher in cats than in dogs and in female than in male animals. According to age, 6-year-old dogs and 9-year-old cats had the highest virus neutralization assay titers. Of the failure cases, 53.0% (53/100) were dogs or cats less than 1 year old. Although the average failure rates in dogs and cats were low at 3.5% and 1.6%, respectively, the factors influencing failure were age and vaccine manufacturer. Therefore, it is necessary to observe the vaccination interval and timing of blood collection after boosting.
Introduction
Rabies is the best-known zoonotic disease and kills infected individuals by impairing central nervous system function; it can be 100% prevented with vaccination [1]. The causative agent of rabies is the rabies virus (RABV), which belongs to the genus Lyssavirus in the family Rhabdoviridae [2]. RABV is transmitted via the bite of an infected animal. The animals responsible for transmitting rabies vary regionally and include dogs, cats, wolves, foxes, mongooses, bats, raccoon dogs, and skunks [3]. Following exposure to the virus, symptoms appear within a period that varies from a few days to over 6 months, depending on the site of the bite. Animals infected with RABV do not show specific clinical signs during the early stages of infection, but behave unusually over time. For example, a calm animal may become aggressive and afraid to drink water, and may try to bite animals or people. Animals showing clinical signs have a 100% mortality rate [4]. Laboratory diagnosis of RABV infection involves identification of antigen in the brain tissue of an animal that is suspected of being infected. The standard diagnostic methods for rabies are the fluorescent antibody test, reverse-transcription polymerase chain reaction, virus isolation using a cell-culture system, and rapid immunochromatographic test kits [5].
Eradicating RABV in animals and humans is a high priority. To achieve this, the World Health Organization, Food and Agriculture Organization of the United Nations, and World Organization for Animal Health (WOAH) have initiated the global project “Zero Human Rabies Deaths by 2030” [6]. As dogs remain the principal source of human rabies infections, inoculation of pet animals with the rabies vaccine and serological investigations to evaluate levels of immunity are of great importance. Methods used to detect antibodies against RABV include fluorescent antibody virus neutralization (FAVN), the rapid fluorescent focus inhibition test, the mouse neutralization test, and enzyme-linked immunosorbent assays [5,7]. Rabies serology provides evidence that an animal has been vaccinated against rabies; very few animals have survived being infected with rabies. Animals with a virus neutralization assay (VNA) titer of 0.5 IU/mL or higher induced by the vaccine are protected against RABV. Among the serological test methods, FAVN and the rapid fluorescent focus inhibition test are recognized as standard tests by the WOAH and World Health Organization, respectively [8]. Therefore, to reduce the incidence of rabies in imported dogs and cats, quarantine authorities in many countries require a rabies serum certificate with VNA titers. Rabies testing of imported animals has been strengthened, but a few imported dogs still have the disease [9]. Despite the increasing number of people and animals traveling abroad together, there is little information on rabies serology for dogs and cats leaving South Korea. In this study, we conducted rabies neutralizing antibody tests on dogs and cats traveling abroad and analyzed the results.
Material and Methods
Cells and virus
BHK-21 (CCL-10; ATCC, USA) cells were grown in Dulbecco’s modified Eagle’s medium supplemented with 5% (v/v) heat-inactivated fetal bovine serum and an antibiotic–antimycotic solution (Gibco, USA). RABV CVS-11 strain (VR959; ATCC) propagated in the BHK-21 cells was used for the FAVN test.
Data and blood collection
Between 2017 and 2021, blood samples were collected from 2,367 dogs and 894 cats preparing to travel abroad. Before collecting blood from the animals, the owner completed a questionnaire on the species, breed, date of birth, sex, and rabies vaccine manufacturer. Information on vaccines other than the rabies vaccine was not collected. A clinic veterinarian checked that dogs and cats over 3 months of age had received the rabies vaccine twice, and blood was collected at 4 weeks after the second vaccination. Blood samples were centrifuged and the sera were stored at −20°C before testing.
FAVN
The FAVN test was performed according to standard WOAH procedures [5], the details of which have been described elsewhere [10]. The VNA titers of the sera samples were expressed in international units (IU)/mL by comparing the results with those of a positive standard purchased from the Nancy Laboratory of the French Agency of Food, Environmental and Occupational Health & Safety.
Statistical analysis
The titers of the rabies serologic tests were expressed in IUs and classified as a pass (≥ 0.5 IU/mL) or failure (< 0.5 IU/mL). The means and distributions of the VNA titers were analyzed using Microsoft Excel 2010 (Microsoft, USA). The differences in VNA titers according to sex were analyzed by two-way analysis of variance, followed by Tukey’s post hoc test using GraphPad Prism ver. 9.3.1 (GraphPad Software, USA). p-values < 0.05 were considered statistically significant.
Results
Serological tests for RABV using FAVN were conducted in samples from 2,367 dogs and 894 cats preparing for overseas trips from South Korea with their owners. The percentages of male dogs and cats tested in this study were 47.3% (1,119/2,367) and 51.5% (460/894), respectively. The ages of the dogs and cats varied from 0.5 to over 12 years. Among the dog and cat breeds, the most common were poodles and Korean domestic short hairs, respectively.
The overall pass rates for RABV were 96.4% (2,281/2,367) in dogs and 98.4% (880/894) in cats (Table 1). No significant differences in the RABV pass rates were observed according to year. The pass rates in dogs ranged from 95.1% (703/739) in 2020 to 99.0% (103/104) in 2018 and those in cats ranged from 96.6% (112/116) in 2017 to 100% (24/24) in 2018, indicating that most dogs and cats had protective antibodies. Dogs and cats had mean rabies VNA titers of 38.3 and 73.0 IU/mL, respectively, indicating that cats had greater immunogenicity to the rabies vaccine than dogs. The respective rabies VNA titers of male and female dogs were 36.2 and 40.7 IU/mL, and those of male and female cats were 71.4 and 74.7 IU/mL, indicating that female dogs and cats had slightly higher immunogenicity to the rabies vaccine (Fig. 1). The most frequent VNA titer ranges were 0.51 to 4.6 IU/mL (26.0%) for dogs and 41.6 to 125.5 IU/mL (32.0%) for cats (Fig. 2).

Pass rates of dogs and cats with rabies virus neutralization assay titers ≥ 0.5 IU/mL according to year
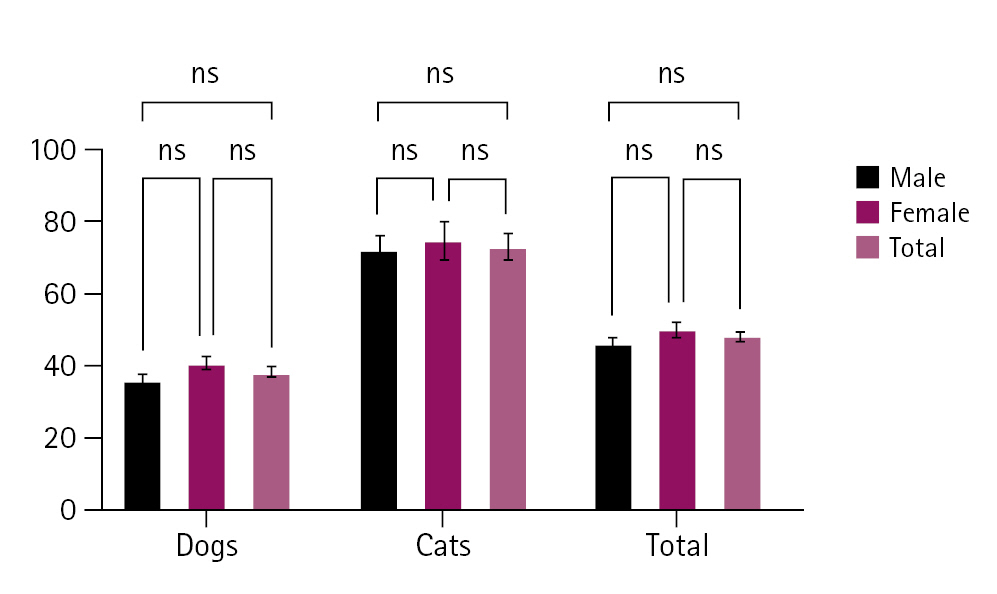
The mean rabies virus neutralization assay (VNA) titers in dogs and cats according to sex. The VNA titers were slightly higher in female than in male dogs and cats. ns, non-significant at p<0.05.

Distribution of rabies virus neutralization assay (VNA) titers in serum from dogs and cats. VNA titers of 0.51 to 4.6 IU/mL were the most frequent in dogs and of 41.6 to 125.5 IU/mL in cats.
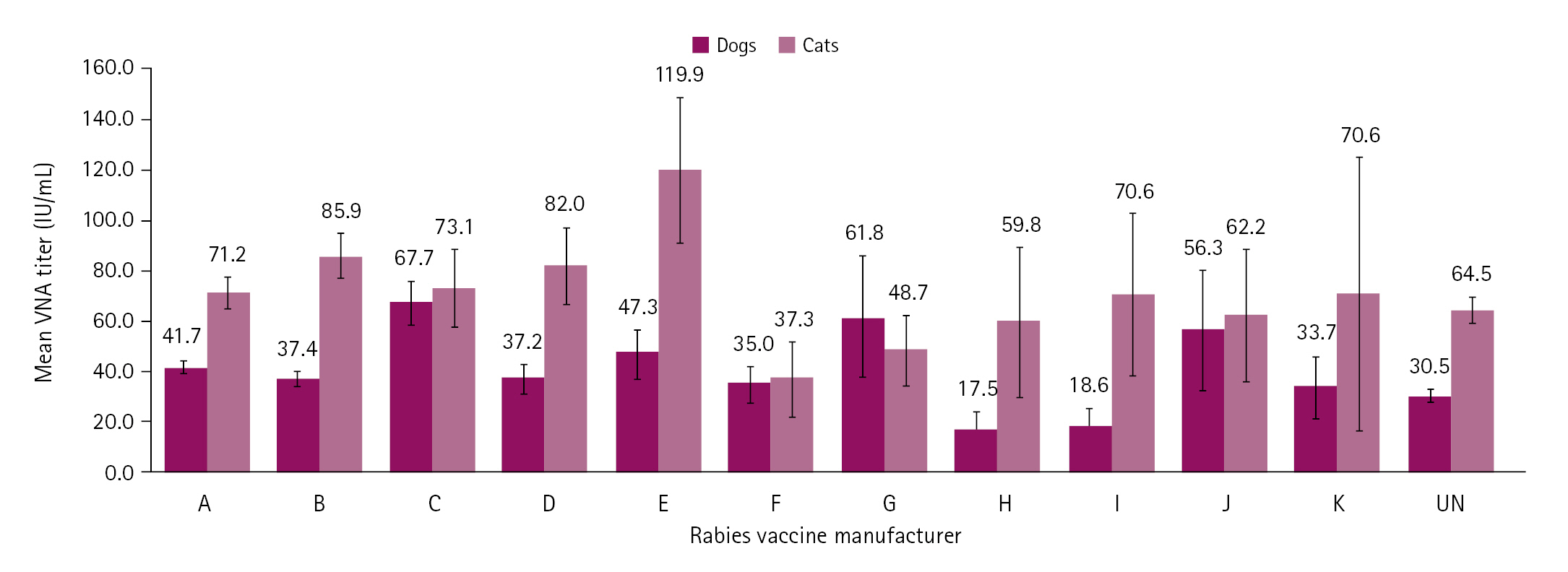
Serological testing of samples for rabies collected between 2017 and 2021 yielded 86 failures in dogs and 14 in cats. Fig. 3A shows the numbers of failure cases based on a VNA titer < 0.5 IU/mL according to the age of the dogs and cats. Dogs and cats < 1 year old accounted for 53.5% (46/86) and 50.0% (7/14) of failure cases, respectively. The mean rabies VNA titers according to the ages of the dogs and cats were 30.4 to 43.8 IU/mL and 29.5 to 113.6 IU/mL, respectively. The highest mean rabies VNA titers were in 6-year-old dogs (43.8 IU/mL) and 9-year-old cats (113.6 IU/mL) (Fig. 3B). Among 11 rabies vaccine manufacturers, the failure rates of vaccines H and K were at 10.5% (2/19) and 20.0% (1/5) in dogs and cats, respectively (Table 2). The mean rabies VNA titers in dogs and cats according to vaccine manufacturer were 17.5 to 67.7 and 37.3 to 119.9 IU/mL, respectively. Rabies vaccines C (67.7 IU/mL) and E (119.9 IU/mL) induced the highest mean rabies VNA titers in dogs and cats, respectively (Fig. 4).
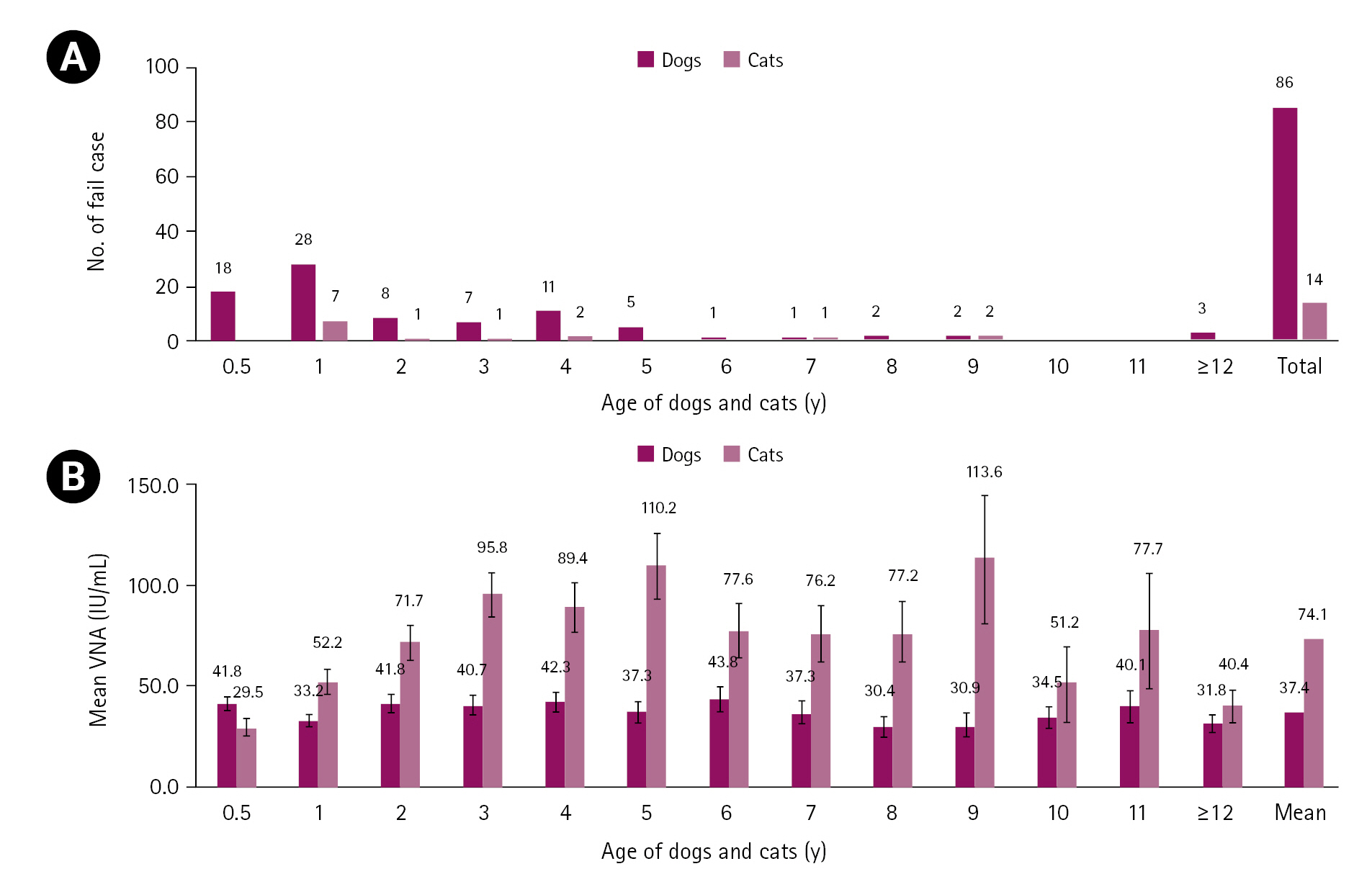
Number of failure cases (A) and mean rabies virus neutralization assay (VNA) titer (B) according to the age of dogs and cats inoculated with the rabies vaccine twice. Dogs and cats < 1 year old had higher failure rates.

No. of failures in dogs and cats with a rabies virus neutralization assay titer < 0.5 IU/mL according to the rabies vaccine manufacturer
Discussion
A person traveling abroad with a companion animal must submit a rabies serum test certificate to the quarantine authorities, who require two things for imported dogs and cats. First, animals intended for import must be immunized with an inactivated rabies vaccine twice after they reach 90 days of age. Second, the animals must have a serum rabies VNA titer of 0.5 IU/mL or higher. The rabies antibody titer test certificate is valid for 2 years from the date of blood collection [11]. To reduce the risk of rabies in imported animals, most countries have implemented these import requirements. In this study, we used the FAVN test to measure rabies VNA titers in the sera of dogs and cats preparing for overseas travel and analyzed the results.
Of the 2,367 dogs examined, 96.4% (2,281/2,367) had rabies antibodies as a result of rabies vaccine inoculation. The pass rate of the dogs in this study in Korea was higher than the 58% rate reported for dogs under field conditions in Bolivia in April 2007 [12] and was similar to the 93.0% rate reported in dogs vaccinated against rabies in France in 2003 [13]. A similar pass rate (97.4%) was reported in military working dogs in South Korea in 2018 [14]. The differences in the pass rate in dogs may be attributed to a number of vaccination and field conditions. The dogs in this study were subjected to serological testing after being vaccinated twice, whereas rabies vaccination of the dogs in Bolivia under field conditions might have been limited for pregnancy and safety reasons. The pass rate (98.4%) of cats was similar to that (97.5%) of cats in France for the period 1993–2002 and that of cats in Switzerland for the period 1999–2007 [13,15].
Similar to reported results [11], we found that the mean rabies VNA titer was higher in cats than in dogs, indicating that cats have a greater immune response to the rabies vaccine than do dogs, which results in increased pass rates in cats. Since 1993, four cases of rabies have been confirmed in feral cats in South Korea [16]. However, there are few reports on serological investigations after rabies vaccination in cats. Therefore, serological information on the rabies vaccine in cats is useful.
There is a relationship between age and failure. Reported factors that significantly affected the failure rate in rabies serology tests were the vaccine type, sampling period, and animal age [17]. Since animal age can significantly influence failure rates, the numbers of failure cases by age were investigated. Samples from Korean dogs and cats collected over a 5-month period had a failure rate of 3.1% (100/3,261). This failure rate is similar to that (5%) reported by laboratories performing rabies serum tests [11]. Nevertheless, young animals (< 1 year) had a high failure rate of 53.0% (53/100), which can be explained by the less-mature immune systems of young animals [17]. Therefore, when vaccinating young animals, it is also necessary to consider keeping the second vaccination interval to 1 month [18].
Along with age, sampling interval, and animal breed, the vaccine manufacturer is another factor that affects failure rates [18,19]. The vaccines from some manufacturers have high failure rates [17]. We investigated the relations of the vaccine manufacturers with failure rates and rabies VNA titers. In this study, animals preparing for overseas travel were vaccinated with 11 types of vaccines, which included three types of inactivated rabies vaccines manufactured in South Korea. Vaccines H and K had failure rates of 10.5% and 20.0% in dogs and cats, respectively. Large numbers of animals will have to be vaccinated with these two vaccines to determine immunogenicity.
In conclusion, this study provides a detailed analysis of VNA titers after rabies vaccination in Korean dogs and cats. More than 96.9% of the dogs and cats evaluated had protective VNA titers against rabies, and this pass rate was significantly higher than that (58%) in dogs surveyed in Bolivia in 2008, indicating that the inactivated rabies vaccines currently in use are effective at inducing protective VNA titers against rabies in dogs and cats. However, dogs and cats less than 1 year old were at high risk of low VNA titers in response to vaccination. This risk can be minimized by adhering to blood collection at 4 weeks after the second vaccination.
Notes
The authors declare no conflict of interest.
Acknowledgements
This work was supported financially by a grant (N 1543085-2017-36-01) from the Animal, and Plant Quarantine Agency, Ministry of Agriculture, Food and Rural Affairs, Republic of Korea.