 |
 |
| Korean J Vet Res > Volume 63(4); 2023 > Article |
|
Abstract
Many scolicidal agents have been used to destroy fertile protoscolices, but these scolicidal agents have side effects, highlighting the need for research on effective and non-toxic replacement scolicidal agents. Gold nanoparticles (AuNPs) are biocompatible and non-toxic. The current study examined the effects of AuNPs in killing the protoscolices of Echinococcus granulosus in vitro using eosin staining. The protoscolices were treated with 0.2, 0.4, 0.8, or 1.0 mg/mL of AuNPs for 15, 30, 45, or 60 minutes. A concentration of 1.0 mg/mL was the most efficient in killing the protoscolices after 60 minutes exposure, reaching 96%, followed by 0.8 mg/mL (84.5%), whereas 0.4 and 0.2 mg/mL of AuNPs achieved a death rate of 76.8% and 68.5%, respectively. The loss of the protoscolices was lower at shorter exposure times with the same concentration of AuNPs and increased as the AuNP concentration was increased at the same exposure time. Significant differences were found between the different groups compared to the control group.
Modern nanotechnologies have helped change many of the medical rules used to prevent, diagnose, and treat diseases. This technology offers new ways for agents to cross the cell membrane and differentiate between target and non-target cells in the human body. Gold nanoparticles (AuNPs) have been studied widely for the treatment of diseases, particularly cancer. AuNPs (5 to 100 nm in size) have been applied since the Middle Ages, which led to the name potable gold [1]. Medically, AuNPs are used in radiomedicine and have a high X-ray absorption coefficient and local surface plasmon resonance [2]. In addition, AuNPs exhibit electronic and optical properties that allow controllable interactions with organic molecules, including electron donor groups [3]. Gold has been used to treat neurological disorders, such as depression, epilepsy, migraine, and gland disorders, such as menopause and sexual dysfunction. On the other hand, the real effect of gold was not understood physiologically for a long time because of the apparent contradiction that gold is a rare metal with virtually no toxicity [4,5]. While gold toxicity has recently been demonstrated, unlike most pharmaceuticals, it is not generally related predictably to the limits it reaches within the body tissues [5].
Cystic hydatidosis is a zoonotic disease of humans. Infection of the intermediate hosts results from ingesting Echinococcus granulosus eggs in contaminated water and food, which develop into the hydatid cysts, mainly inside the liver or other body tissues. Dogs, wolves, and foxes are considered the definitive hosts of E. granulomas [6,7]. This disease is characterized by the absence of pathological symptoms in the initial stages of infection. With time, the cysts grow in size inside the organ, leading to pressure on the affected organ and its neighboring organs [6,8]. Patients typically exhibit an inflammatory reaction. In advanced cases, the hydatid cyst may obstruct the function of the affected part. On the other hand, various immune reactions, such as urticaria, edema, and anaphylactic shock, occur when the cyst ruptures and the hydatid fluid is released, possibly resulting in death [9-11].
The complete failure of surgical therapy was one of the reasons that prompted researchers to use many other methods to treat it, such as chemotherapy, immunotherapy, radiotherapy, and treatment using medicinal plants. Recently, some nanomaterials have been studied to determine their effect on the vitality of protoscolices outside the living body. This study evaluated the scolicidal effect of AuNPs against protoscolices of hydatid cysts.
Hydatid cysts were collected from naturally infected sheep from the Diwaniyah abattoir in Diwaniyah Province, Iraq. The cysts were washed from the outside with tap water to remove blood and suspended matter accumulated during slaughter and placed inside a clean container. They were then transferred to the parasitology laboratory at the Department of Biology, Faculty of Education, University of AL-Qadisiyah. The protoscolices were aspirated under sterile situations and saved in sterile tubes. The vitality of the protoscolices was assessed by taking 1.5 mL of the protoscolices suspension and staining with 1% eosin. The solution was shaken well, and a drop was taken from it and examined under a microscope. The live protoscolices appeared green compared to the dead protoscolices, whose membranes had been penetrated by the dye and dyed red [12].
AuNPs were obtained as ready-made oxide as a gold powder with a purity of 99.9% and a particle size of 30 to 60 nm (Sky Spring Nanoparticles Inc.). The AuNPs were prepared from a stock solution (2 g of AuNPs per liter of distilled water) and sterilized in an autoclave. The solution was mixed using an ultrasonic homogenizer for 15 minutes, after which 0.2, 0.4, 0.8, and 1 mg/mL colloids were prepared and kcept in the refrigerator until use.
In this step, 1.5 mL of the suspension of the protoscolices was transferred to 20 tubes. The tubes were then treated with 0.2, 0.4, 0.8, or 1 mg/mL of gold nanoparticles for 15, 30, 45, or 60 minutes. The arithmetic average of the protoscolices vitality was calculated under the above conditions, as evidenced by the permeability of eosin dye. The protoscolices were examined by optical microscopy. The live and dead protoscolices were counted based on the dyeing of protoscolices with the eosin dye [13].
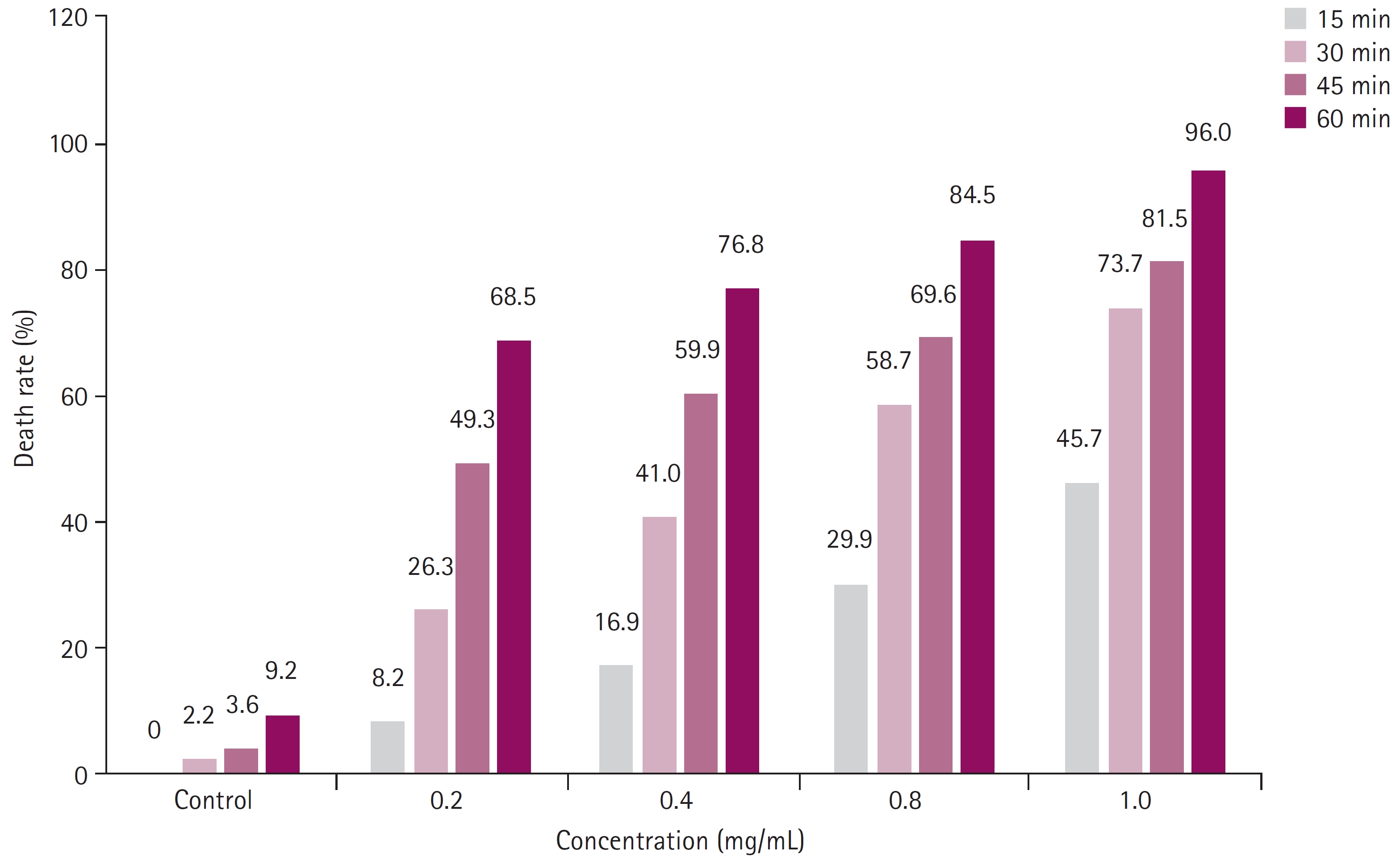
The percentage of protoscolices destruction increased as the concentration and duration of exposure to AuNPs increased (Table 1 and Fig. 1). The percentage destruction was 68.5%, 76.8%, 84.5%, and 96.0% at 0.2, 0.4, 0.8, and 1.0 mg/mL, respectively, after a 60 minutes treatment, representing the highest percentage of protoscolices mortality compared to the protoscolices mortality treated with the same concentrations for shorter periods. The lowest mortality was observed in the group treated for 15 minutes: 8.2%, 16.9%, 29.9%, and 45.7% at 0.2, 0.4, 0.8, and 1.0 mg/mL, respectively. The mortality rate increased with time. The death rate after 30 minutes was 26.3%, 41.0%, 58.7%, and 73.7% at 0.2, 0.4, 0.8, and 1.0 mg/mL, respectively. After 45 minutes of treatment, the death rate of the protoscolices was 49.3%, 59.9%, 69.6%, and 81.5% at 0.2, 0.4, 0.8, and 1.0 mg/mL, respectively. In contrast, the control group showed a 0%, 2.2%, 3.6%, and 9.2% death rate at 15, 30, 45, and 60 minutes, respectively (p<0.05). Overall, the AuNPs caused significant morphological changes to the protoscolices, as shown in Fig. 2.
Hydatid disease is a widespread disease with an estimated prevalence of 5% to 10% [14,15]. According to the World Health Organization, Iraq has a high endemicity of hydatid cyst disease. Clinical studies have shown that treatment using drugs does not always kill the protoscolices [16], and surgical methods are not devoid of risks, such as the rupture of hydatid cysts and spillage of their contents [17]. Therefore, research is continuing to discover effective and safe methods. AuNPs were used in this study as a scolicidal agent because of the presence of previous studies that demonstrated their effectiveness against various parasites, such as protozoa comprising [18] Toxoplasma gondii, Trypanosoma spp., Leishmania spp. [19], and Cryptosporidium spp.; helminths comprising trematodes (Schistosoma spp.) [20] and cestodes (Raillietina spp.) and vectors containing mosquitoes [21].
Eosin stain penetration into the primary primates was used to verify the vitality of protoscolices because eosin penetration is a permeable process related to the nature of the permeability of the membrane. In contrast, live protoscolices retain their natural green color [22,23]. Four concentrations of AuNPs were used (0.2, 0.4, 0.8, and 1.0 mg/mL) with 0.9% physiological saline used as the control group. The results indicated that the death rate of the protoscolices increased directly as the concentration and duration of exposure to the AuNPs increased.
The death rates of the protoscolices exposed 0.2, 0.4, 0.8, and 1.0 mg/mL for 60 minutes were highest at 68.5%, 76.8%, 84.5%, and 96%, respectively. By contrast, the lowest percentage of protoscolices death after 15 minutes of treatment was 8.2%, 16.9%, 29.9%, and 45.7%, respectively. This result is consistent with ├ćolak et al. [24], who reported that AuNPs have a scolicidal effect and can treat cysts associated with the bile ducts. In addition, another study [25], which used four concentrations of AuNPs, reported that AuNPs have promising results as a scolicidal agent for cystic hydatid cysts.
The 1 mg/mL concentration was the optimum because it led to a 96% death rate after 60 minutes owing to the inhibitory effect of gold nanoparticles, which may have affected the physiology of the protoscolices through its effect on enzymes or stopping the cell metabolism cycles that occur within the protoscolices. The AuNPs lead to paralysis and then the death of worms. The authors attributed this to changes in the enzymatic activity of the worms [26] in addition to its effect on a group of protozoa [27-29] and the morphological changes in the protoscolices. The morphological distortions of the protoscolices exposed to gold nanoparticles were attributed to the loss of the plasma membrane function or defects in the equilibrium of ions on both sides of the membrane plasma.
Acknowledgments
The author is thankful to the management of the University of Al-Qadisiyah for their continuous encouragement and support. Funding was provided by author, assist prof. Sadiya Aziz Anah.
Fig.┬Ā1.
Death rates of protoscolices treated with gold nanoparticles (0.2, 0.4, 0.8, and 1 mg/mL) in anisochrony (15, 30, 45, and 60 minutes).
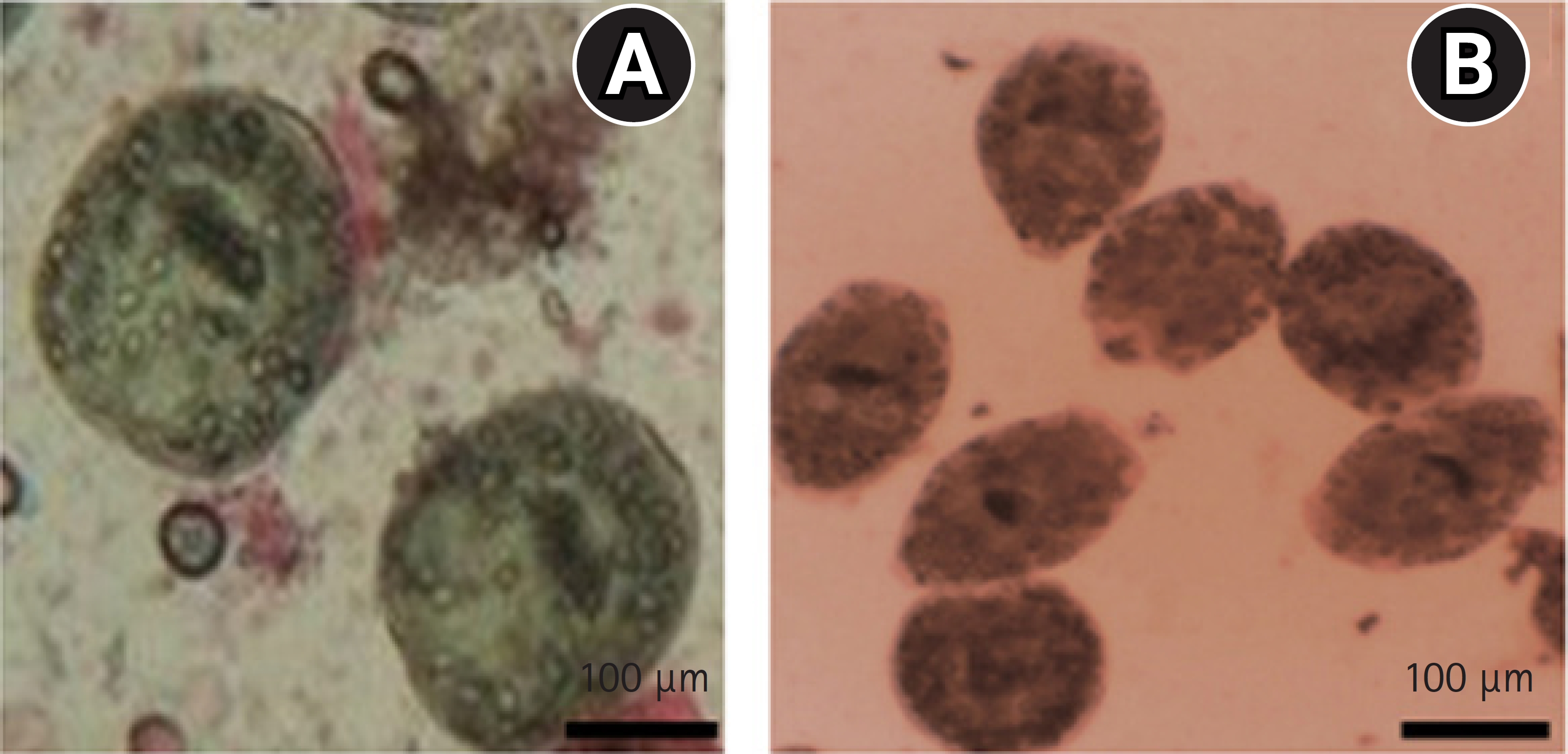
Fig.┬Ā2.
Protoscolices. (A) Live (nontreated). (B). Protoscolices were treated with different concentrations of gold nanoparticles using eosin stain. Scale bar = 100 ╬╝m.
Table┬Ā1.
Effect of different concentrations of AuNPS on the mortality rates of protoscolices and for different time periods in vitro
References
1. Liz-Marz├Īn LM. Gold nanoparticle research before and after the Brust-Schiffrin method. Chem Commun (Camb) 2013;49:16-18.


2. Bai X, Wang Y, Song Z, Feng Y, Chen Y, Zhang D, Feng L. The basic properties of gold nanoparticles and their applications in tumor diagnosis and treatment. Int J Mol Sci 2020;21:2480.



3. Dumur F, Dumas E, Mayer CR. Functionalization of gold nanoparticles by inorganic entities. Nanomaterials (Basel) 2020;10:548.



4. Richards DG, McMillin DL, Mein EA, Nelson CD. Gold and its relationship to neurological/glandular conditions. Int J Neurosci 2002;112:31-53.


5. Merchant B. Gold, the noble metal and the paradoxes of its toxicology. Biologicals 1998;26:49-59.


6. Dvorak G, Rovid-Spickler A, Roth JA. Handbook for Zoo┬Łnotic Diseases of Companion Animals. Center for Food Security and Public Health, Lawa State University, Ames, 2008.
7. Dandan IS, Soweid AM, Abiad F. Hydatid cysts. Med J 2007;1-20.
8. Chen YC, Yeh TS, Tseng JH, Huang SF, Lin DY. Hepatic hydatid cysts with superinfection in a non-endemic area in Taiwan. Am J Trop Med Hyg 2002;67:524-527.


9. Eckert J, Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev 2004;17:107-135.



10. Gottstein B. Hydatid Disease. In: Powderly Cohen J, eds. Infection Disease. 2nd ed. pp. 1-6, Mosoby, London, 2003.
12. Kanaan MH, Anah SA, Jasim GA, Ghasemian A. In-vitro protoscolicidal and immunomodulatory effects of Cinnamomum camphora and Ziziphora tenuior against Echinococcus granulosus protoscolices. Rev Med Microbiol 2021;32:45-50.

13. Al-Rubaie SH. The effect of some plant extracts on the weakening of protoscolices outside and inside the body in the albino mouse [dissertation]. College of Science, University of Baghdad, Baghdad: 1999.
14. Craig PS, McManus DP, Lightowlers MW, Chabalgoity JA, Garcia HH, Gavidia CM, Gilman RH, Gonzalez AE, Lorca M, Naquira C, Nieto A, Schantz PM. Prevention and control of cystic echinococcosis. Lancet Infect Dis 2007;7:385-394.


15. Budke CM, Deplazes P, Torgerson PR. Global socioeconomic impact of cystic echinococcosis. Emerg Infect Dis 2006;12:296-303.



16. Gil-Grande LA, Rodriguez-Caabeiro F, Prieto JG, S├Īnchez-Ruano JJ, Brasa C, Aguilar L, Garc├Ła-Hoz F, Casado N, B├Īrcena R, Alvarez AI, Dal-R├® R. Randomised controlled trial of efficacy of albendazole in intra-abdominal hydatid disease. Lancet 1993;342:1269-1272.


17. Gottstein B, Eckert J, Woodtli W. Determination of parasite-specific immunoglobulins using the ELISA in patients with echinococcosis treated with mebendazole. Z Parasitenkd 1984;70:385-389.


18. Jain S, Melo TG, Dolabella SS, Liu J. Current and emerging tools for detecting protozoan cysts and oocysts in water. TrAC Trends Anal Chem 2019;121:115695.

19. Sazgarnia A, Taheri AR, Soudmand S, Parizi AJ, Rajabi O, Darbandi MS. Antiparasitic effects of gold nanoparticles with microwave radiation on promastigots and amastigotes of Leishmania major. Int J Hyperthermia 2013;29:79-86.

20. Pissuwan D, Gazzana C, Mongkolsuk S, Cortie MB. Single and multiple detections of foodborne pathogens by gold nanoparticle assays. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2020;12:e1584.


21. Moodley JS, Krishna SB, Pillay K, Sershen F, Govender P. Green synthesis of silver nanoparticles from Moringa oleifera leaf extracts and its antimicrobial potential. Adv Nat Sci Nanosci Nanotechnol 2018;9:015011.

22. Risan FA. A study of the possibility of attenuation of protoscolices of E. granulosus using different types of laser rays [dissertation]. College of Science, University of Baghdad; Baghdad: 1994.
23. Al-Abboudi A K J. Weakening of the of protoscolices E. granulosus using some plant extracts [dissertation]. College of Science, University of Baghda; Baghdad: 2001.
24. ├ćolak B, Aksoy F, Yavuz S, Demircili ME. Investigating the effect of gold nanoparticles on hydatid cyst protoscolices under low-power green laser irradiation. Turk J Surg 2019;35:314-320.



25. Barabadi H, Honary S, Ali Mohammadi M, Ahmadpour E, Rahimi MT, Alizadeh A, Naghibi F, Saravanan M. Green chemical synthesis of gold nanoparticles by using Penicillium aculeatum and their scolicidal activity against hydatid cyst protoscolices of Echinococcus granulosus. Environ Sci Pollut Res Int 2017;24:5800-5810.


26. Tikariha S, Singh S, Banerjee S, Vidyarthi AS. Biosynthesis of gold nanoparticles, scope and application: a review. Int J Pharm Sci Res 2012;3:1603-1615.
27. Varela-Aramburu S, Ghosh C, Goerdeler F, Priegue P, Moscovitz O, Seeberger PH. Targeting and inhibiting Plasmodium falciparum using ultra-small gold nanoparticles. ACS Appl Mater Interfaces 2020;12:43380-43387.



- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 595 View
- 19 Download
- ORCID iDs
-
Sadiya Aziz Anah

https://orcid.org/0000-0001-5550-4036 - Related articles


 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



