Ventricular septal defect (VSD) occurs when the ventricular septum fails to develop properly during cardiac embryonic development, leaving an opening in the heart [
1]. It is one of the most common congenital heart diseases in cats [
2]. VSDs can be classified into 4 types based on their location, perimembranous, muscular, supracristal, and inlets [
3]. Perimembranous VSDs, located just below the aortic valve in the left ventricular outflow tract, are the most common type in small animals [
1,
3].
The prognosis of VSD largely depends on the size of the defect [
4]. In isolated, restrictive, and small VSDs (less than 40% of aortic diameter and with shunt velocity greater than 4.5 m/s), most patients are asymptomatic and have a good prognosis [
4]. However, in large non-restrictive VSDs (more than 60% of aortic diameter and with shunt velocity less than 4.0 m/s), prognosis can be guarded, and congestive heart failure is a common complication [
4]. Medical treatment or surgical closure can be attempted, but when these fail, Eisenmenger syndrome may develop [
4].
While spontaneous closure of VSD is common in humans, it is very rare in small animals [
5,
6]. To the best of our knowledge, only 5 cases of spontaneous closure in dogs and none in cats have been reported, respectively [
6]. Although VSDs can be diagnosed with computed tomography or magnetic resonance imaging, the most commonly used methods are auscultation and transthoracic echocardiogram [
7]. Echocardiogram was used in previous reports to diagnose spontaneous closure in dogs; we relied on the same to diagnose spontaneous closure in our case [
6].
A 5-month-old male British Shorthair, weighing 2.5 kg, was brought to Seoul Animal Heart Hospital following the detection of a heart murmur during a routine vaccination appointment at a different hospital. Physical examination revealed that the cat was bright, alert, and responsive. A grade 1 to 2 systolic murmur was detected at the region of the aortic valve during auscultation. Femoral pulses were strong and synchronous, and 6-lead electrocardiography revealed a heart rate of 140 beats/min, left axis deviation and intermittent ventricular septal defect. Respiratory rate, pattern, and sounds were normal. The mucous membranes were pink, and the capillary refill time was less than 2 seconds. Doppler systolic blood pressure was 120 mmHg. The remaining indicators measured during the physical examination were normal, and the heart size, pulmonary vessels, and bronchial tubes appeared normal on chest radiography. No other anomalies were detected.
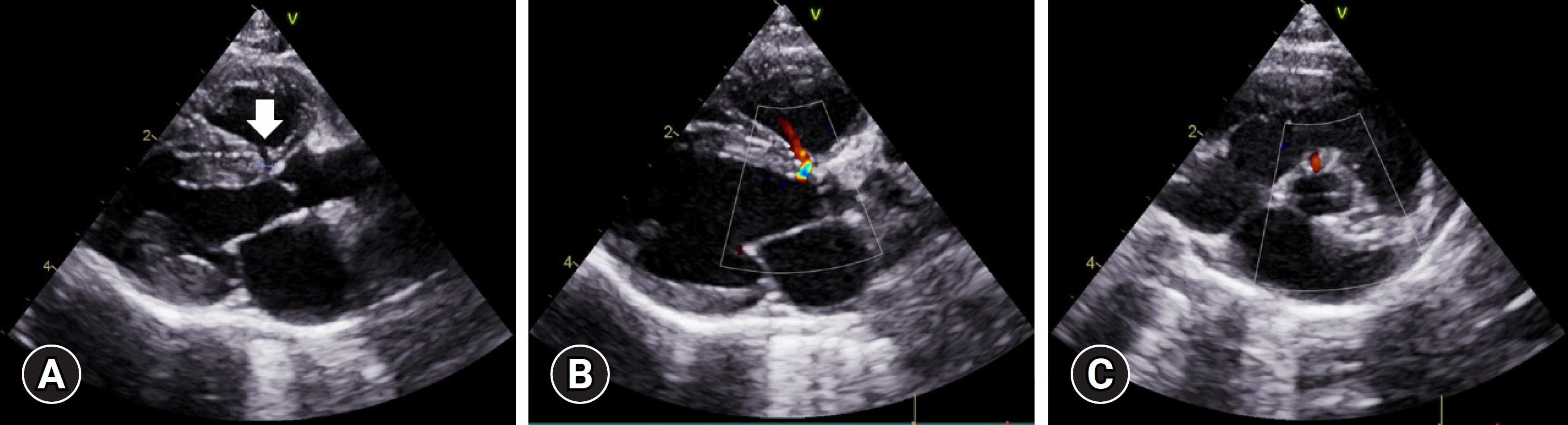
Two-dimensional echocardiography performed by a board-certified cardiologist revealed a small (1.18 mm), isolated, and restrictive perimembranous VSD located immediately below the aortic valve at the interventricular septum in the right parasternal 5-chamber and short-axis views (
Fig. 1A). In the same 2 views, color Doppler revealed a Doppler signal at identical sites (
Fig. 1B and
C). The pulse-waved Doppler method was used to confirm left-to-right shunting in the right parasternal 5-chamber view. No right ventricular enlargement or remodeling was observed. Pulmonary artery was normal in size without signs of pulmonary arterial hypertension. The left ventricle was normal in thickness, with no myocardial thickening of the papillary muscle. The cat was thus diagnosed with perimembranous VSD without secondary cardiac remodeling. As the cat was asymptomatic and exhibited normal heart function, no treatment was initiated, and follow-up monitoring for 6 months to 1 year was recommended.
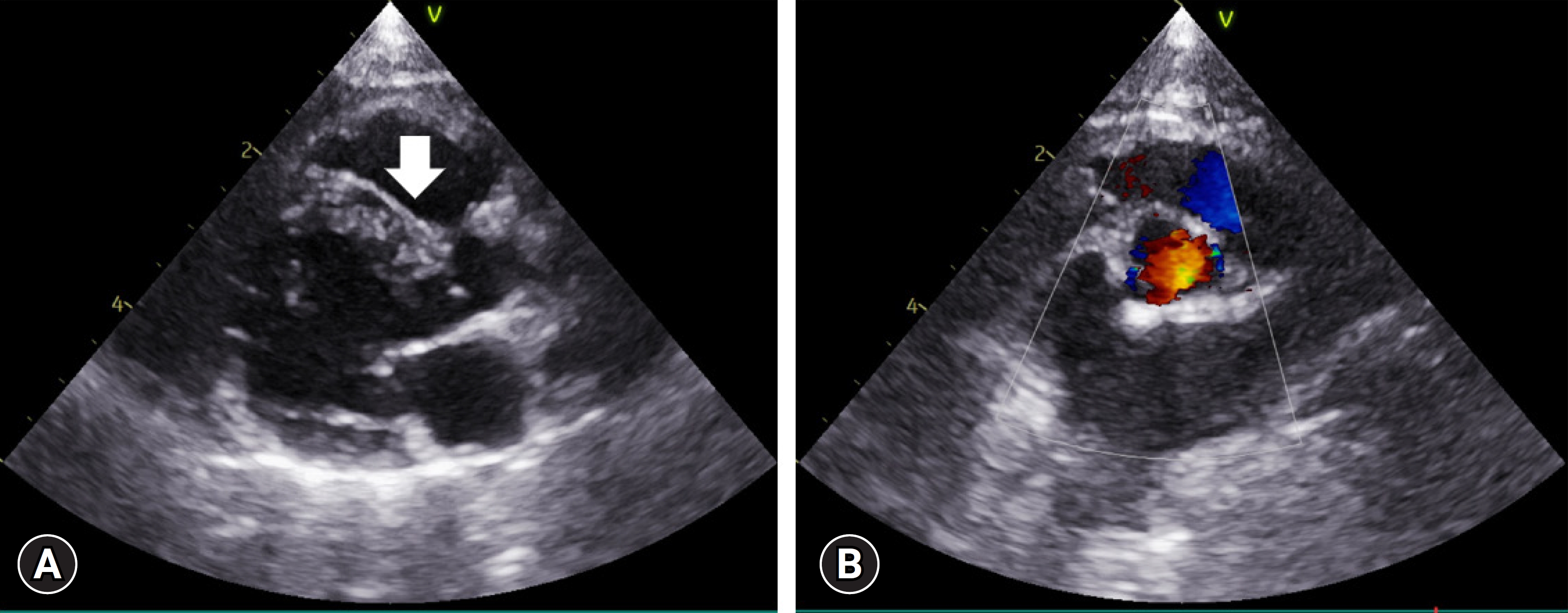
On several subsequent examinations, no significant changes in the heart were observed, and no notable symptoms were reported by the owner before re-assessment at 2 years and 5 months of age. As the cat was fiercely uncooperative and the owner refused sedation, the physical examination could not be properly conducted. Two-dimensional echocardiography revealed no defect at the previously observed VSD site in the right parasternal 5-chamber view (
Fig. 2A). Color Doppler at the same site showed no color signals at the interventricular septum. Similarly, no signals were observed using pulsed-wave Doppler. To confirm the findings, the ventricular septum was assessed in various views, including the right parasternal short-axis and left parasternal 5-chamber views; no defects were identified (
Fig. 2B). No anomalies were observed in any other non-cardiac regions and no murmur was audible on auscultation anymore. Thus, we concluded that the cat’s perimembranous VSD had closed spontaneously.
The cat described in this report was diagnosed with perimembranous VSD at 5 months of age. Follow-up monitoring revealed spontaneous closure of the VSD by the age of 29 months. Such spontaneous closure is relatively common in humans but extremely rare in dogs, and no such cases have been reported in cats.
The VSD clinical course varies markedly according to the presence of residual defects and spontaneous closure [
4]. In case of residual defects, patients may be asymptomatic or exhibit a spectrum of symptoms, including Eisenmenger syndrome, depending on the defect location, size, pulmonary vascular resistance, and the extent of cardiac remodeling due to chronic volume or pressure overload [
4]. The cat referred here, exhibited a perimembranous-type VSD with a considerably small opening (1.18 mm), resulting in an absence of secondary cardiac remodeling. The velocity of the left-to-right shunt was as low as 2 to 3 m/s, suggesting a poor prognosis given the low-pressure difference between the left and right ventricles. However, the measured value may have been lower than the actual value, given the extreme uncooperativeness of the cat and the substantially small size of the VSD. Although the velocity (2-3 m/s) of the shunt was not accurately measured, we can still conclude that the VSD in this case was a left-to-right shunt. While it is unsatisfactory that we could not obtain a reliable result from the Doppler echocardiography, we were able to clearly identify the absence of blood flow between the VSD foramen and the right ventricle 24 months later.
Spontaneous VSD closure is a critical prognostic indicator in humans that must be considered from the time of initial diagnosis [
8]. Miyake et al. [
5] reported that among all VSDs, spontaneous closure occurred in 48% of patients with or without congestive heart failure, with a mean age of 6.9 years, 72% of whom had no congestive heart failure. In veterinary medicine, reports on spontaneous closure are rare. Five cases of spontaneous closure have been reported in dogs [
6,
9], but none in cats. Considering that VSD is one of the most common congenital heart disorders in cats, this is rather surprising [
2].
Studies concerning factors that promote spontaneous closure of VSD in humans are relatively abundant. Such studies have reported frequent spontaneous closures for relatively small openings (3-6 mm) before or immediately after 1 year of age [
10]. Rates of spontaneous closure also vary according to defect type, reaching 69% for muscular types and 28.5% for perimembranous types [
11]. Although the reason for discrepancies between types remains unknown, the higher rate of spontaneous closure for muscular types may be related to their simpler structure and the higher level of growth-related activity in muscles than ligaments [
12]. In perimembranous cases, spontaneous closure occurs due to the attachment of the multiple tricuspid valves and aortic valvular prolapse. In muscular cases, closure occurs as the muscular septum grows and fibrous tissues are generated [
13]. Here, the causes of spontaneous closure were likely related to the young age of the cat at the time of detection, the small size of the opening, and the lack of congestive heart failure, in line with the factors previously reported to contribute to spontaneous closure [
13]. Similar findings have been reported in dogs; however, unlike in humans, most cases of spontaneous VSD closure in dogs involve perimembranous defects, as observed in the cat of the current report [
6]. While this implies that spontaneous closure rates are higher for perimembranous defects in animals than in humans, statistical evidence to support this notion cannot be obtained due to the considerably low number of reports.
The reason for the difference in the rate of spontaneous closure between humans and animals remains unknown. Nonetheless, some speculations can be made. While development progresses over a 20-year period in humans, the equivalent stage of growth in small animals is only 1 year, meaning that spontaneous closure may occur more quickly in these animals [
14]. Additionally, as the heart rate is higher in small animals, blood flow may prevent the generation of new tissues [
15]. Lastly, given that VSD is a congenital heart disorder, early detection is critical in humans. However, echocardiography examinations are not routinely performed in young dogs and cats, contributing to a lower level of detection. Based on these findings, future studies should incorporate necropsy, histochemical testing, and echocardiography to increase the evidence for spontaneous VSD closure in small animals.
This study had some limitations. First, the exact time of spontaneous closure was difficult to determine. Follow-up monitoring from 6 months to 1 year had been recommended to the owner, as the VSD was initially considered clinically insignificant. Additionally, the focus of echocardiography was the detection of secondary cardiac remodeling, as spontaneous closure had not been anticipated.
In cats and dogs, VSD is a relatively common congenital heart disorder, though only 5 cases of spontaneous VSD closure in dogs have been reported [
6]. This case describes a narrow perimemberanous VSD in a cat with no cardiac remodeling, resulting in very low clinical risk and no requirement of any cardiac medications. However, it is a significant case demonstrating that spontaneous VSD closure is possible in cats, similar to humans and dogs.








 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



