|
 |
| Korean J Vet Res > Volume 63(4); 2023 > Article |
|
Abstract
Acknowledgments
Fig.┬Ā1.
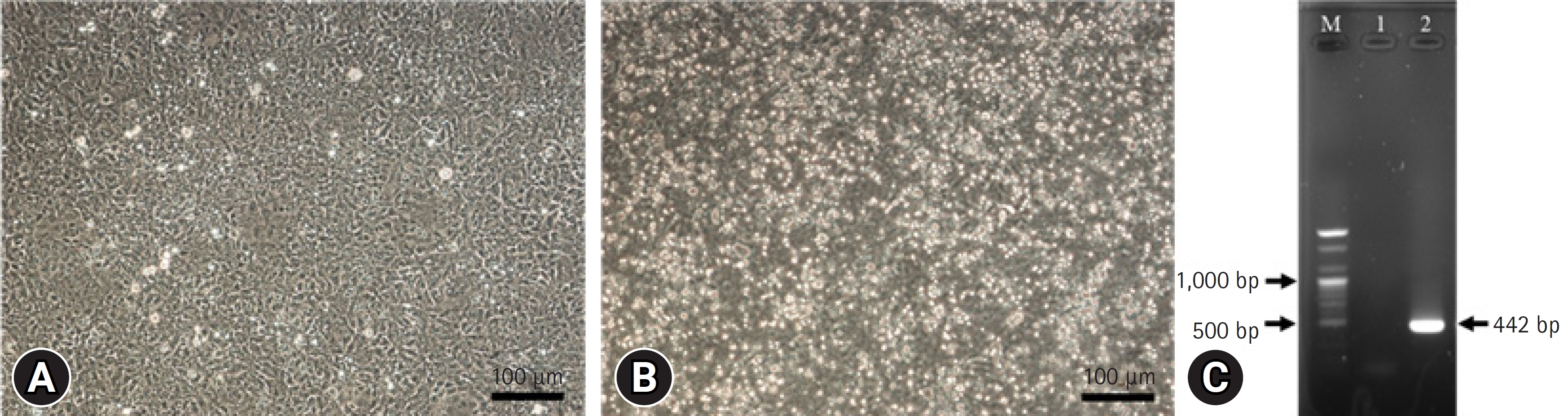
Fig.┬Ā2.
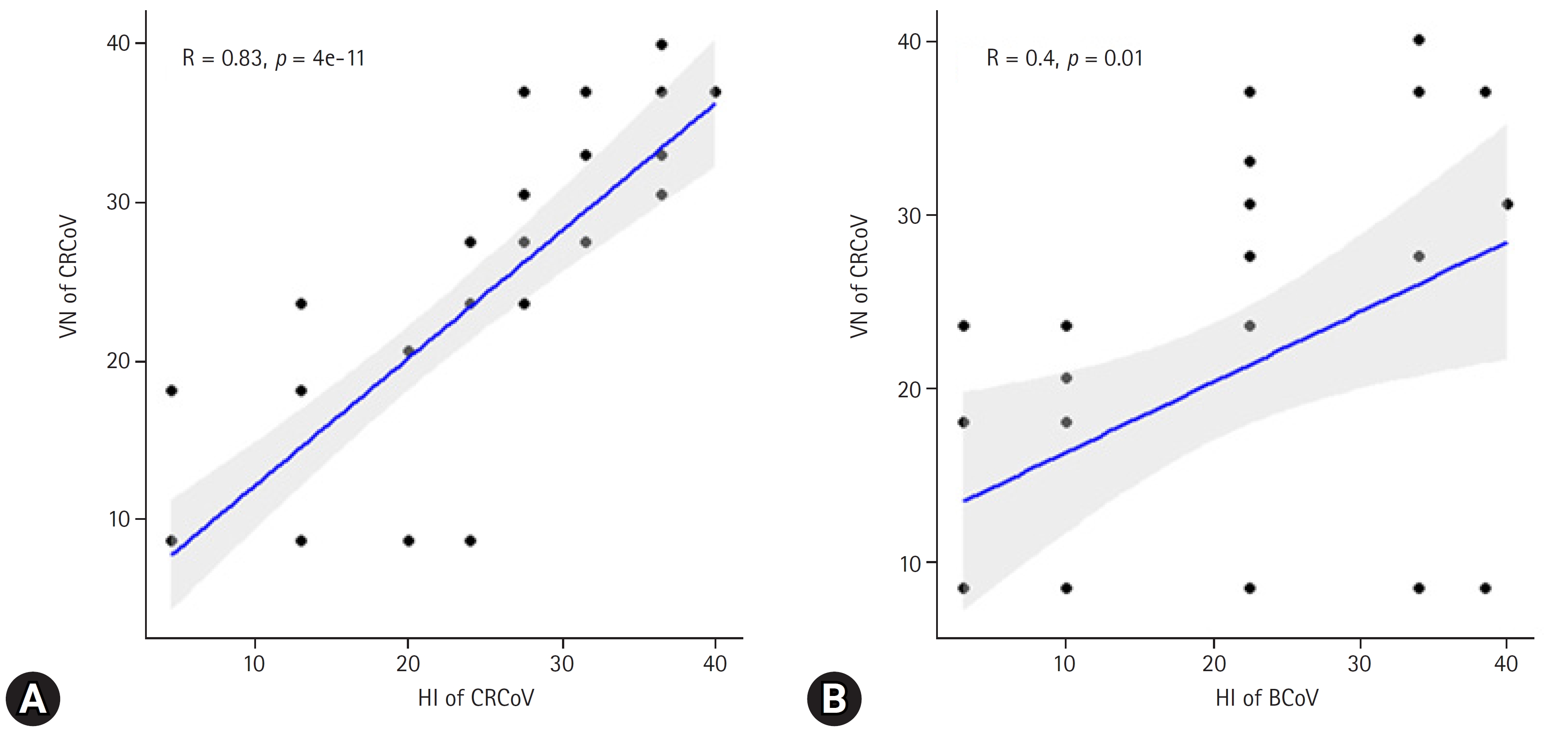
Fig.┬Ā3.
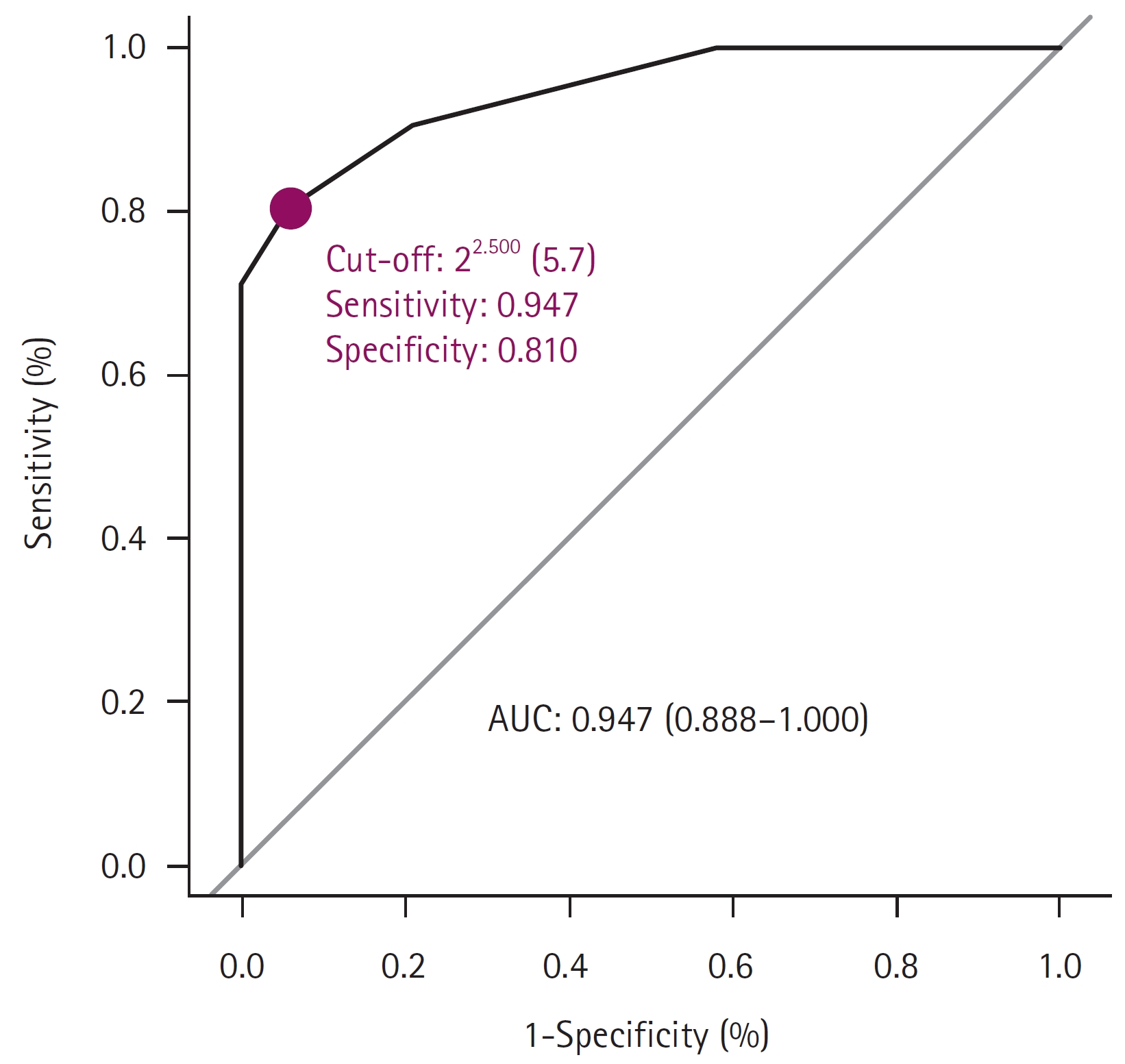
Fig.┬Ā4.
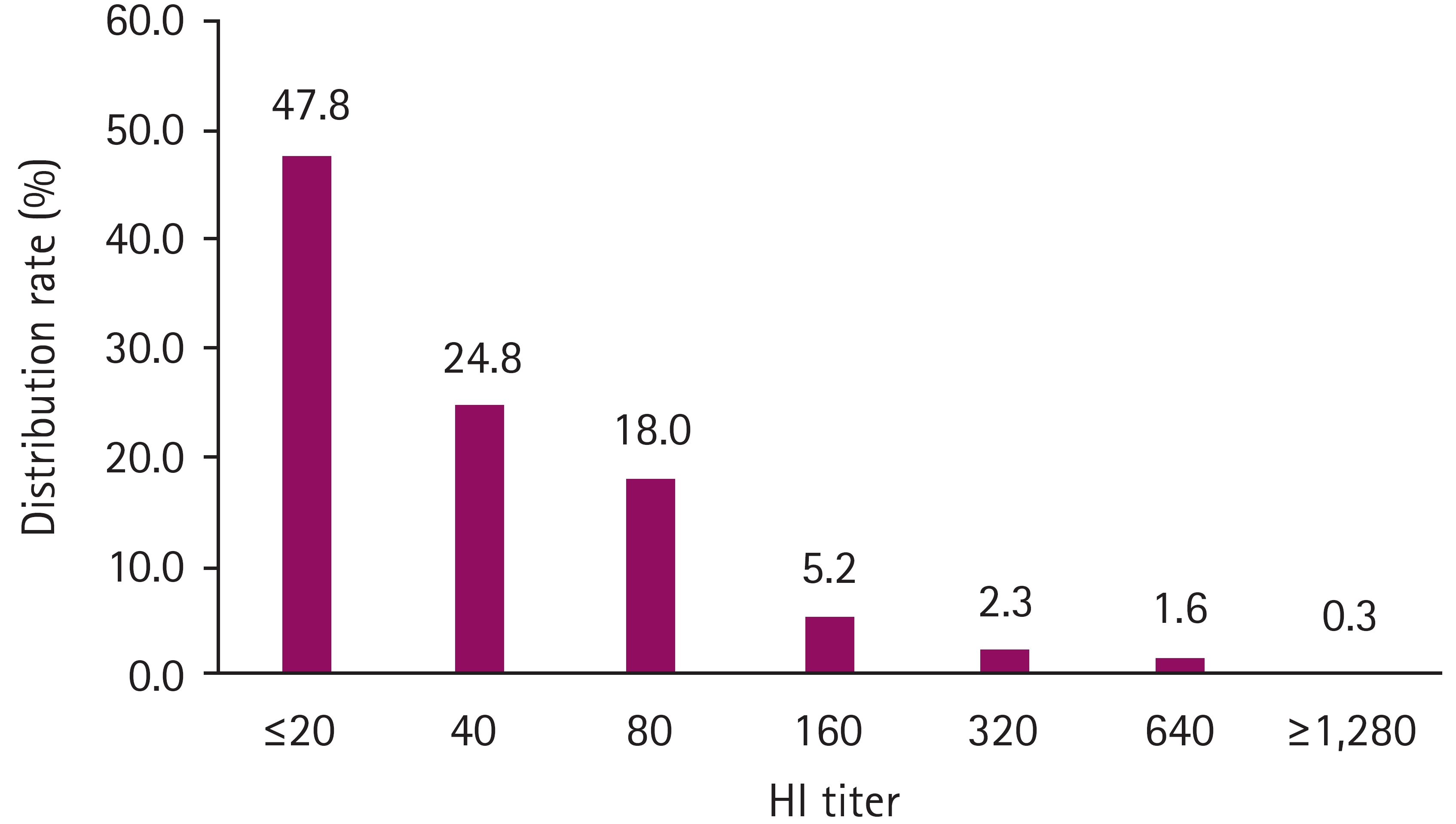
Table┬Ā1.
Table┬Ā2.
HI, hemagglutination inhibition; VN, virus neutralization; CRCoV, canine respiratory coronavirus.
Sensitivity (%) = ([number of positives in both tests) / (number of positives in VN test]) ├Ś 100; Specificity (%) = [(number of negatives in both tests) / (number of negatives in the VN test)] ├Ś 100; Accuracy (%) = [(number of positives in both tests + number of negatives in both tests) / (total number of samples)] ├Ś 100.
Table┬Ā3.
| Variable | Positive/total (%) | OR (95% CI) | p-value* |
|---|---|---|---|
| Sample collection year | < 0.001 | ||
| ŌĆā2022 | 57/96 (59.4) | 1.00 (ref.) | |
| ŌĆā2021 | 50/93 (53.8) | 0.79 (0.44-1.42) | |
| ŌĆā2020 | 29/96 (30.2) | 0.29 (0.16-6.15) | |
| ŌĆā2019 | 64/98 (65.3) | 1.28 (0.71-2.31) | |
| Age (y) | 0.007 | ||
| ŌĆāŌēż 1 | 50/114 (43.9) | 1.00 (ref.) | |
| ŌĆā1-2 | 50/107 (46.7) | 1.12 (0.66-1.91) | |
| ŌĆā3-5 | 58/87 (66.7) | 2.54 (1.43-4.59) | |
| ŌĆāŌēź 6 | 42/75 (56.0) | 1.62 (0.90-2.94) | |
| Sex | 0.93 | ||
| ŌĆāMale | 108/205 (52.7) | 1.00 (ref.) | |
| ŌĆāFemale | 92/178 (51.7) | 0.96 (0.64-1.43) | |
| ŌĆāTotal | 200/383 (52.2) |
References
- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 801 View
- 40 Download
- ORCID iDs
-
Lee-Sang Hyeon

https://orcid.org/0000-0002-7608-156XDong-Kun Yang

https://orcid.org/0000-0001-5765-3043Yu-Ri Park

https://orcid.org/0000-0002-2829-4368Hye Jeong Lee

https://orcid.org/0000-0003-2044-6176Ha-Hyun Kim

https://orcid.org/0000-0001-6473-0035Bang-Hun Hyun

https://orcid.org/0000-0002-3429-3425 - Related articles


 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print