Canine T-cell lymphoma encompasses various lymphoid neoplasms arising from T lymphocytes [
1,
2]. Large cell types are known to have an aggressive clinical course, while small cell T-cell lymphoma has an indolent form referred to as T-zone lymphoma (TZL) [
1-
4]. Immunophenotyping using flow cytometric analysis (FCA) or immunohistochemistry is helpful in the diagnosis of TZL [
4-
6].
As TZL has indolent clinical features, conservative management without chemotherapeutic agents is indicated in most cases, and its overall prognosis is better than that of other high-grade T-cell lymphoma [
1,
3,
4]. However, chemotherapy may be considered when a patient is symptomatic or in a progressive state [
1]. To date, chemotherapy, including CHOP-based chemotherapy (cyclophosphamide, doxorubicin, vincristine, and prednisone), chlorambucil, and prednisolone, has been described to treat canine TZL [
1,
3]. Studies have shown that there is no significant difference in survival time among the different chemotherapy protocols, ranging from 10 months to 24.5 months [
3,
7]. This report presents the diagnosis and chemotherapy of two dogs with TZL and assesses the applicability of chlorambucil and prednisolone in canine TZL.
Case 1. A 10-year-old spayed female Shih-tzu dog presented with palpable bilateral submandibular masses. The shape of submandibular masses was round to oval with 2.0 to 2.5 centimeter. On physical examination, generalized peripheral lymphadenopathy including bilateral submandibular, prescapular and popliteal lymph nodes was found, and marked leukocytosis (60.78 ├Ś 10
9/L; reference range, 5.05-16.76 ├Ś 10
9/L) and lymphocytosis (43.13 ├Ś 10
3/┬ĄL; reference range, 1.05-5.10 ├Ś 10
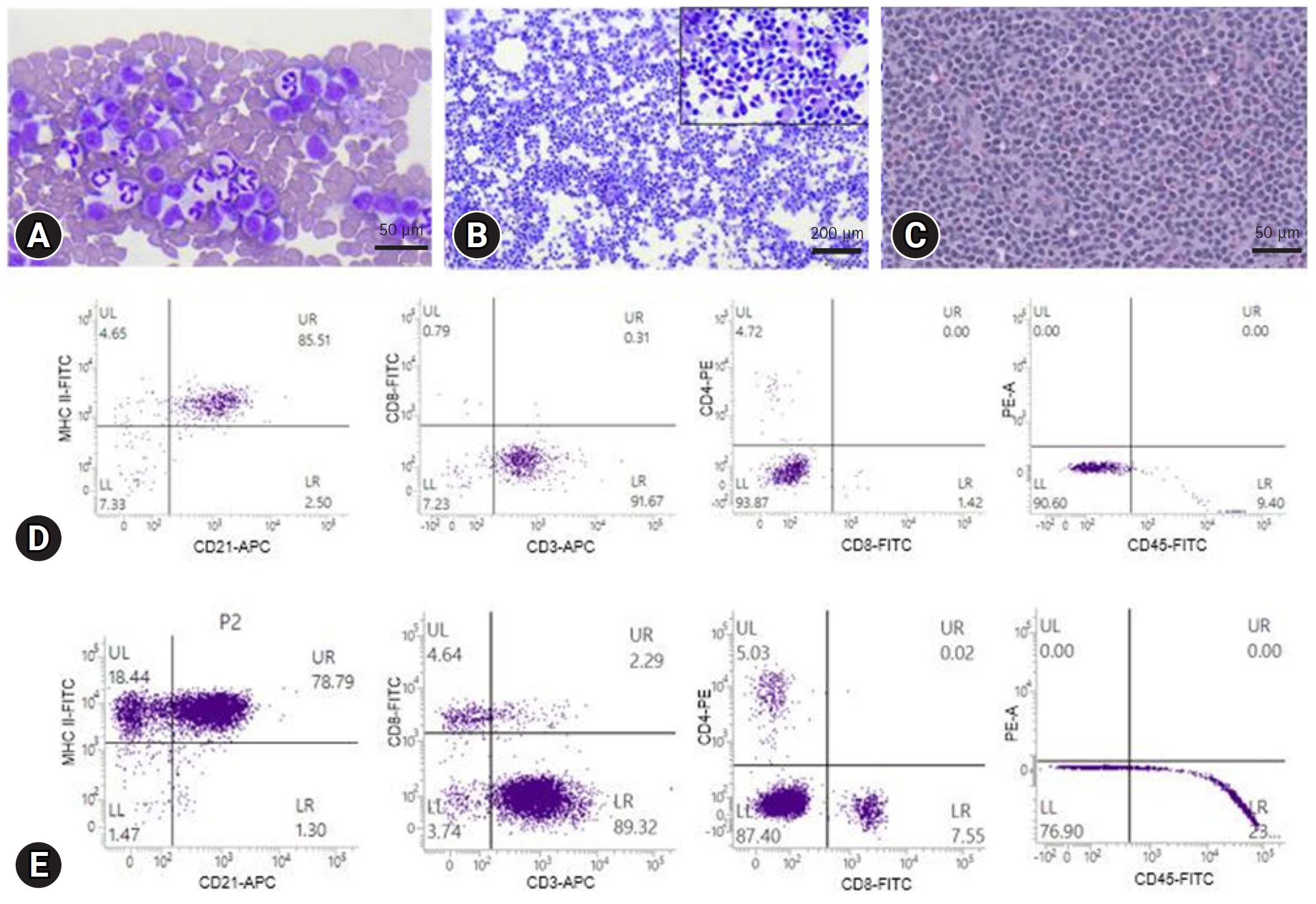
3/┬ĄL) were detected on blood analysis (Procyte Dx; IDEXX Inc., USA). Fine-needle aspiration (FNA) of submandibular lymph nodes revealed a homogeneous population of small to intermediate-sized lymphocytes with contorted cytoplasmic borders known as ŌĆ£hand-mirrorŌĆØ appearance. Small lymphocytes were predominantly observed on blood smear examination (
Fig. 1A and
B).
Polymerase chain reactions for antigen receptor rearrangement (PARR) (Korea Vet Lab., Korea) and FCA (Veterinary Medical Teaching Hospital, Seoul National University, Korea) were performed using peripheral blood and lymph node aspirates for immunophenotyping (
Table 1). The right popliteal lymph nodes were surgically resected for histopathological examination. The PARR assay showed T-cell clonality, and FCA detected lymphocytes with CD3+/CD4-/CD8-/CD21+/CD45- and high MHC II expression (BD FACSVerse; BD Biosciences, USA). Histopathologically, the normal nodal architecture was found to be replaced by sheets of neoplastic lymphocytes (
Fig. 1C-
E). Consequently, the patient was diagnosed with stage Va TZL depending on the World Health Organization staging scheme for lymphoma in domestic animals [
8].
Despite the indolent nature of TZL, chemotherapy with chlorambucil (4 mg/m
2, per os [PO], every 24 hours [q24h], Leukeran Tab.; Aspen, Australia) and prednisolone (1 mg/kg, PO, q24h, Solondo; Yuhan Pharma, Korea) was administered because of the heavy burden of lymphocytosis [
3].
One month after initiating chemotherapy, submandibular and prescapular lymph nodes decreased in about half the size, and the left popliteal lymph node was rarely palpated. In addition, leukocytosis (20.43 ├Ś 109/L) and lymphocytosis (11.46 ├Ś 103/┬ĄL) improved. The initial chlorambucil dose was maintained and prednisolone was tapered to 0.5 mg/kg q24h.
After two months of treatment, both leukocyte (15.88 ├Ś 109/L) and lymphocyte (1.37 ├Ś 103/┬ĄL) count normalized. The decrease in size of the submandibular lymph nodes was maintained and prescapular and left popliteal lymph nodes were rarely palpated. The prednisolone dose was tapered to 0.5 mg/kg q48h.
During periodic monitoring, there were no additional changes in the size of the peripheral lymph nodes, and the lymphocyte count was maintained in the reference range without adverse events during chemotherapy. The dog remained stable for about 10 months since the start of treatment without disease progression, but the chemotherapy had been discontinued after having discussion with the owner.
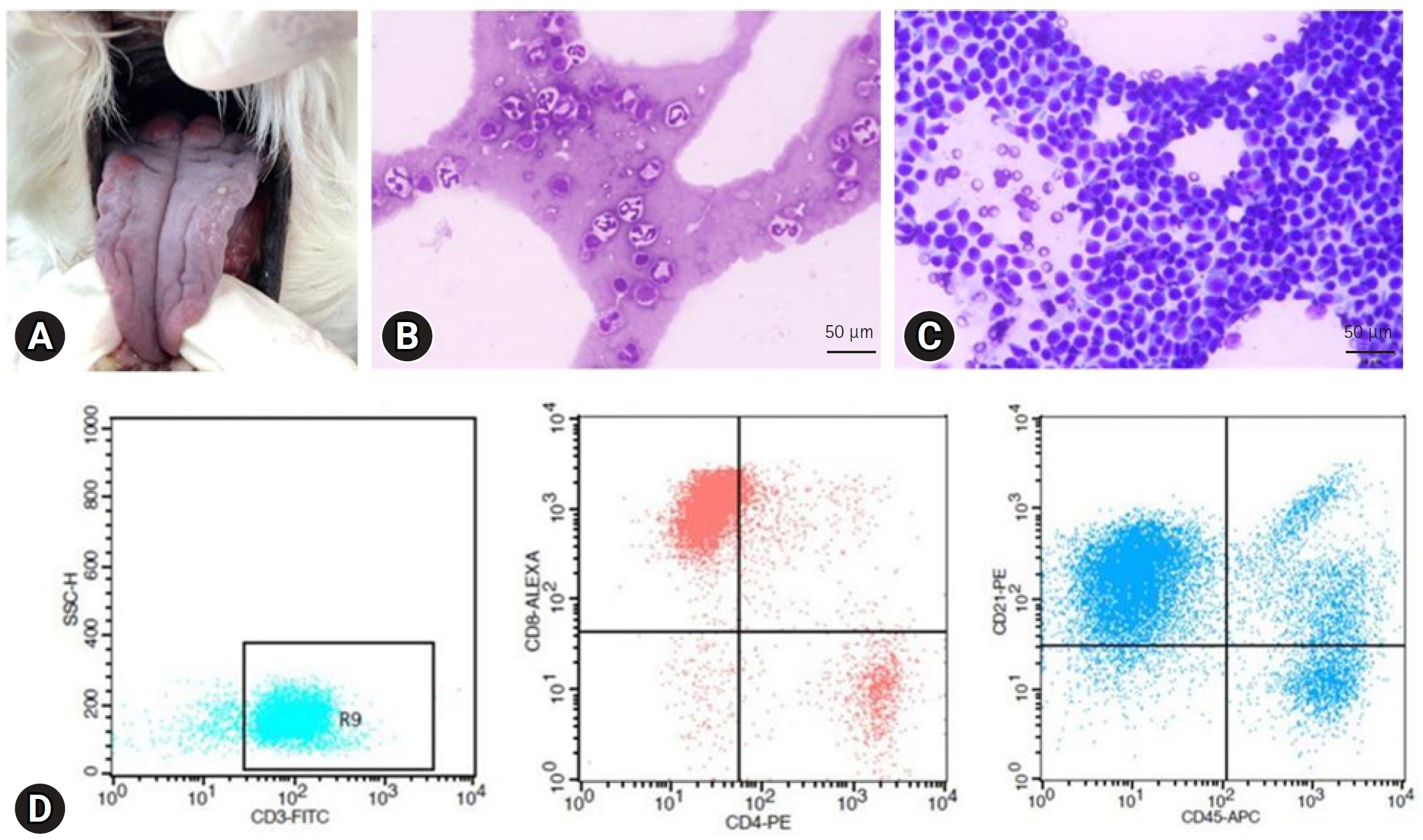
Case 2. An 11-year-old spayed female Maltese dog presented with nodular masses on the tongue. Physical examination revealed generalized peripheral lymphadenopathy including bilateral submandibular, prescapular, and popliteal lymph nodes and these were smooth and rubbery with round to oval shape on palpation. The gross lesions on the tongue appeared as raised and red nodular masses (
Fig. 2A). In addition, there was lymphocytosis (6.60 ├Ś 10
3/┬ĄL) on blood analysis (Procyte Dx). FNA cytology of the lymph nodes was performed, and it revealed small to intermediate lymphocytes presenting ŌĆ£hand-mirrorŌĆØ appearance. Small lymphocytes were observed in peripheral blood smears (
Fig. 2B and
C).
FCA was performed (Animal Medical Center, Gyeongsang National University, Korea) on lymph node aspirates, and the results were consistent with TZL, including CD45 negativity and CD3/CD4/CD8/CD21 positivity (
Fig. 2D). Although the lingual lesions have not been evaluated cytologically or histologically, regarding the fact that TZL can involve other organs and referring to the previous study describing clinical characteristics and diagnosis of canine lingual TZL cases [
9], the patient was diagnosed with stage Vb TZL due to tongue involvement and peripheral lymphocytosis [
8]. Despite tumor related clinical signs were absent, we decided to start the chemotherapy including chlorambucil (3 mg/m
2, PO, q24h) and prednisolone (1 mg/kg, PO, q24h) due to the possibility of manifesting clinical symptoms with oral lesions as the disease progressed. After one week of treatment, the lingual lesions improved. The prednisolone dose was tapered to 0.5 mg/kg q24h, and after three weeks of therapy, to 0.3 mg/kg q24h. Chlorambucil was maintained at the same prescribed dose.
Two months after treatment initiation, peripheral lymphadenopathy resolved, and lymphocyte count (4.10 ├Ś 103/┬ĄL) was in reference range. The lingual lesions varied with the dosage of prednisolone without aggravation; therefore, we increased the dosage to 1 mg/kg q24h and maintained it thereafter. The patient experienced no adverse effects during chemotherapy. The patient responded well to chemotherapy and was evaluated as stable, and there is no disease progression over a one-year follow-up period.
TZL is an indolent form of T-cell lymphoma usually associated with a long survival time without presenting clinical signs [
1-
4]. Immunophenotyping is critical for the diagnosis of TZL. The characteristics of the TZL immunophenotype have been well described, including the loss of CD45 expression [
1,
3-
6]. In our cases, the results of immunophenotyping were consistent with those of TZL, and small- to intermediate-sized lymphocytes were found on cytological examination. However, in one study, medium-to large-sized T-cell lymphomas showed CD45 negativity, and the patient had a poor response to chemotherapy and died 7.5 months after diagnosis, which is shorter than the reported survival time for TZL [
1,
3,
7,
10]. Therefore, diagnosing TZL only through immunophenotyping could lead to misdiagnosis, and multimodal diagnostic tests, including cytology or histopathology, are required to make a definitive diagnosis of TZL.
Owing to its slowly progressive clinical course, conservative management with careful monitoring is often recommended for TZL [
1-
4]. However, in TZL cases with rapid disease progression, having clinical signs, organ dysfunction due to infiltration, and marked peripheral lymphocytosis, treatment could be considered [
1]. Flood-Knapik KE et al reported that patients who present peripheral lymphocyte count of > 9.212 ├Ś 10
3/┬ĄL show shorter median survival time than those with lower counts [
3]. The patient in case 1 showed marked peripheral lymphocytosis (43.13 ├Ś 10
3/┬ĄL). In case 2, the patient had oral lesions that could cause clinical symptoms related to the space-occupying effect [
9]. Thus, systemic therapy may be reasonable in both these cases. However, there are no set criteria for indication of chemotherapy or treatment guidelines of TZL to date, only suggested conditions for chemotherapy exist [
1]. Therefore, further studies are required to establish the standard for treatment of TZL.
TZL usually presents in a nodal form; however, extranodal involvement has also been reported [
2,
11]. In case 2, the patient was diagnosed with TZL through FNA and FCA of the lymph nodes and had gross lesions on the tongue. The clinical characteristics observed in our cases were similar to those reported for canine lingual TZL [
9], including the features of lesions on the tongue, peripheral lymphadenopathy and/or lymphocytosis, and immunophenotyping results. In the aforementioned study, although most patients were asymptomatic at initial presentation, some patients had clinical signs associated with the oropharyngeal region. In a previous retrospective study, dogs showing clinical signs had shorter survival times than those without, and the authors suggested that this might behave as a negative prognostic factor, like in high-grade lymphoma [
2]. Therefore, if TZL has organ involvement even without presenting clinical signs, preemptive chemotherapy could be considered as the organ-related clinical signs may appear with the progression of the disease.
Chlorambucil, an alkylating agent, has been widely used as a metronomic dosing agent in small animal medicine [
12]. Chemotherapy for canine TZL has been reported, including prednisone, a CHOP-based protocol, lomustine, and chlorambucil, and the outcomes for treatment with chlorambucil for TZL are variable including complete remission [
1,
3,
9,
11]. Conservative protocols, such as low doses of chlorambucil or cyclophosphamide, are more recommended than aggressive chemotherapy regarding the indolent nature of disease [
13]. In one study, the survival time between the CHOP protocol and chlorambucil showed no significant difference at 19.3 and 24.5 months, respectively [
3]. In addition, it has been reported that the CHOP protocol is associated with severe adverse effects in 45.6% of cases, whereas metronomic chlorambucil causes minor events [
12,
14]. A case series demonstrated that chlorambucil and prednisolone successfully controlled TZL [
11]. Although we did not achieve complete remission, the patients showed improvement with metronomic chlorambucil, including resolution of peripheral blood lymphocytosis and a decrease in lymph node size without deterioration of the disease condition, while there were no chemotherapy-related adverse events. Thus, chemotherapy with chlorambucil and prednisolone is an appropriate treatment choice for dogs with TZL.
The lack of more advanced evaluation of lingual lesion is limitation in this study. Different conditions from TZL, such as inflammation, could develop tongue lesions like in the patient, and depending on the nature, they may have responded to chlorambucil or prednisolone, or both. However, considering the ability of TZL to involve other organs and clinical characteristics described in the previous report of canine lingual TZL cases [
9], lesions on the tongue in this patient are likely related to the TZL involvement.
In conclusion, conservative management is usually indicated for canine TZL considering its indolent characteristics. However, chemotherapy can be administered in certain cases presenting organ involvement and disease progression. Chemotherapy using chlorambucil and prednisolone is one of the treatment choices, and metronomic chemotherapy appears to be well tolerated by canine patients.












 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



