Follicular fluid-derived extracellular vesicles improve in vitro maturation and embryonic development of porcine oocytes
Article information
Abstract
To optimize the most efficient method for porcine in vitro maturation (IVM), we compared the effects of supplementing extracellular vesicles (EVs) derived from porcine follicular fluid (pFF). The cumulus oocyte complexes were grouped into 4 groups with different supplementations as following: pFF (G1), pFF-depleted EVs (G2), EVs (G3) and control (G4) groups. After IVM with different supplementations, maturation rates and the developmental competences of porcine oocytes and blastocyst development were investigated. Additionally, glutathione (GSH) and reactive oxygen species (ROS) levels were measured in mature oocytes. The EVs were isolated and characterized with cryo-TEM and nanoparticle tracking analysis. The pFF significantly affected the maturation rate, whereas the presence of EVs did not show notable difference in the maturation rates. Although there were numerical increases in the measured parameters in EV and pFF-depleted EVs groups, no significant differences were observed between them. The EV group showed similar oocyte maturation rate for both positive and negative control groups. The GSH was not different among the groups, but ROS levels were significantly lower in pFF-supplemented group when compared with other groups with the highest level in the control group. G2 group wasn’t significantly different G1 and G3 group. G3 group wasn’t significantly different from G2 and G4 group. This suggests that EVs in IVM medium which probably effected partially to protect against oxidative stress and potentially enhance the quality of oocytes. This study indicates that the EVs in pFF play a significant role in improving the efficiency of oocyte maturation in porcine.
Introduction
In vitro maturation (IVM) of oocytes and development of embryos are essential processes in various medical fields, such as in vitro fertilization for infertile couples and production of animal models for disease research [1]. However, the rates of oocyte maturation and embryo development in vitro are significantly low compared to those observed in vivo [2]. To overcome these challenges, various studies and experiments have been conducted at different stages of IVM and in vitro culture (IVC) [3,4]. The ovarian follicle plays an important role in regulating the hormones necessary for oocyte maturation [5,6]. Additionally, it provides essential nutrients and oxygen required for oocyte maturation [7]. Recent study has shown that the ingredients present in follicular fluid (FF) can affect oocyte maturation and quality [8].
Extracellular vesicles (EVs) are nanovesicles enclosed by a lipid bilayer and can be released from all living cells [9]. They contain many biologically active molecular substances such as proteins, mRNAs and miRNAs, which play active roles in regulating different physiological events [10,11]. The EVs play important roles in intercellular communication and are involved in a wide range of physiological and pathological processes [12].
The EVs are biogenic molecules known for their high stability, and compared to other additives, they enhance the efficiency of IVM. Therefore, this study aimed to investigate the effects of EVs, which have recently gained significant attention in the field of porcine follicular fluid (pFF), on the maturation and development of oocytes in vitro. Finally, we explored the possibility of using EVs as a natural additive for IVM.
Materials and Methods
Chemicals
All the chemicals were acquired from Sigma-Aldrich (USA) unless otherwise specified.
FF preparation and EVs isolation and characterization
Pig ovaries, contained in approximately 36°C to 38°C saline solution, are transported to the laboratory. We obtained EVs from the FF of 3 to 8 mm follicles. FF was aspirated using an 18-gauge needle and a 10 mL disposable syringe. Aspirated FF was centrifuged at 503 g for 30 minutes and filtered using 0.2 μm filter to remove cells. The filtrate was centrifuged at 120,000 g for 70 minutes, and the isolated pellets were stored at -80°C [13]. The supernatant was used as pFF-depleted EVs. The EVs were characterized using nanoparticle tracking analysis (NTA) for determining the size and concentration. For further characterization, the EVs were visualized through cryogenic transmission electron microscopy (cryo-TEM). The EVs was vitrified using Quantifoil R1.2/1.3 Cu 300 grids and Vitrobot mark IV. Cryo-TEM images were captured using a Glacios microscope [14].
IVM oocytes and EVs supplementation
FF was collected by aspirating 3 to 8 mm-diameter ovarian follicles using an 18-gauge needle and a syringe. Cumulus oocyte complexes (COCs) were washed with HEPES-buffered Tyrode’s medium containing polyvinyl alcohol (TLH-PVA). Only COCs with many layers of compact cumulus cells and even cytoplasm were used and washed 3 times with TLH-PVA.
The COCs were then divided into different groups and cultured for 20 to 22 hours in respective media with gonadotropin, in presence of 5% CO2 in humidified atmosphere at 39°C. They were then transferred to media without gonadotropin and cultured for an additional 20 to 22 hours [15]. The media for all the groups contained TCM-199 (Gibco; Thermo Fisher Scientific Inc., USA) supplemented with 0.6 mM cysteine, 0.91 mM sodium pyruvate, 75 µg/mL kanamycin, 10 ng/mL epidermal growth factor, and 1 µg/mL insulin. In addition, 10 IU/mL hCG and 10 IU/mL PMSG were added to the IVM medium during the initial 20 to 22 hours.
The pFF group served as the positive control group (G1), in which 10% pFF was added to the IVM medium. The pFF-depleted EVs (G2) group was formulated by adding the supernatant after ultracentrifugation during the EV isolation step. The EV group (G3) comprised the EVs derived solely from pFF and pFF was not supplemented in negative control group (G4).
Measurement of oocyte glutathione and reactive oxygen species levels
To measure glutathione (GSH) and reactive oxygen species (ROS) in mature oocytes, oocytes (n = 10, 3 replicates) at the MII stage with the first polar body were selected from each group after 40 to 44 hours of IVM. For measuring GSH levels, 4-chloromethyl 6.8-difluoro-7-hydroxycoumarin (CellTracker Blue, CMF2HC; Invitrogen Corporation, USA) was used, and for ROS measurement, 2',7'-dichlorodihydrofluorescein diacetate (H2DCFDA; Invitrogen Corporation) was used. Oocytes were cultured in TLH-PVA containing 10 µM CellTracker Blue and 10 µM H2DCFDA in the dark for 30 minutes [16,17]. Afterward, they were washed in Dulbecco's phosphate-buffered saline supplemented with 0.1% polyvinyl alcohol (PVA). The GSH and ROS levels were measured using an epifluorescence microscope with excitation at 370 nm for both. ImageJ software (National Institutes of Health, USA) was used to measure the fluorescence intensity.
Parthenogenetic activation after IVM and embryo IVC
After 44 hours IVM culture, cumulus cells were removed using 0.1% hyaluronidase and gentle pipetting. Denuded oocytes were washed in TLH-PVA, and only matured oocytes with the first polar body were used. The oocytes were gradually equilibrated in activation medium for parthenogenetic activation. For activation, the oocytes were activated with a double direct current (DC) pulse of 120 V for 60 μs with a BTX electro cell manipulator (2001). The oocytes were activated with a double DC pulse of 120 V for 60 μs. Electrically activated oocytes were washed twice in TLH medium, thrice in fresh porcine zygote medium-5 (PZM-5) and placed into 25 μL PZM-5 droplets (10 embryos per drop). Activated oocytes were incubated at 39°C in a humidified atmosphere of 5% O2, 5% CO2 and 90% N2 for 7 days. Cleavage was assessed on the day-2 and the blastocyst formation was assessed on the day-7 of IVC.
Statistical analysis
The statistical analysis was conducted utilizing IBM SPSS Statistics ver. 22.0 software (IBM Corp., USA). One-way analysis of variance and LSD post hoc test were performed for mean comparison. The values were represented as mean ± standard errors of the mean. Statistical significance was considered when the p-value was less than 0.05.
Results
EVs isolation and characterization
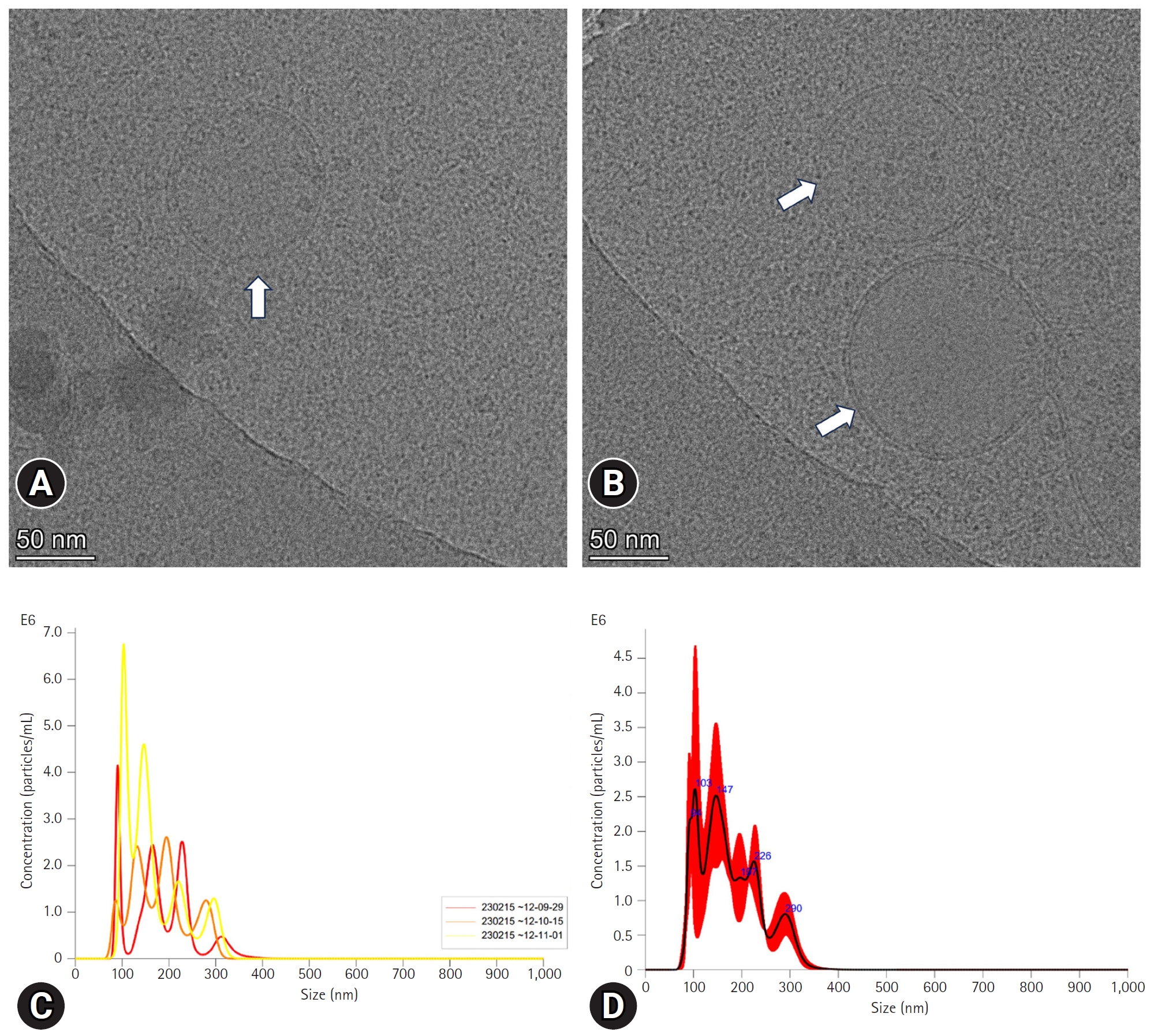
The pFF-derived EVs were first characterized using NTA to measure the size and concentration of the particles. Results showed that they have an average size of 176.7 ± 5.5 nm, and the concentration of those particles was 3.01 × 108 ± 1.95 × 107. Furthermore, for EV characterization (a second method), we measured the shape and size of the particles using cryo-TEM. The results revealed the presence of lipid bilayer vesicles with an average size of < 150 nm (Fig. 1A and B).
Comparison of maturation rates based on the presence of pFF or EVs
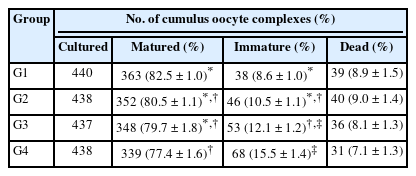
Results showed that the maturation rate of the pFF group (G1) was 82.5% ± 1.0%. G2 (pFF-depleted EVs) had a maturation rate of 80.5% ± 1.1%, the EV group (G3) had a maturation rate of 79.7% ± 1.8%, and the control group (G4) showed a maturation rate of 77.4% ± 1.6% (Table 1). A significant difference was observed depending on the presence of pFF. No significant differences between G1 and G2 groups while there was a tendency toward numerical improvement in the measured parameters in both groups when compared with G3 and G4.
Measurement of oxidative stress in matured oocytes
No significant differences were observed among all the groups in terms of GSH levels (Fig. 2). In contrast, ROS levels was reduced in G1 and G2 groups when compared with G3 and G4 groups. G1group differed significantly from G3 and G4 groups, whereas the G2 group showed significant differences only with the G4 group (Fig. 3).
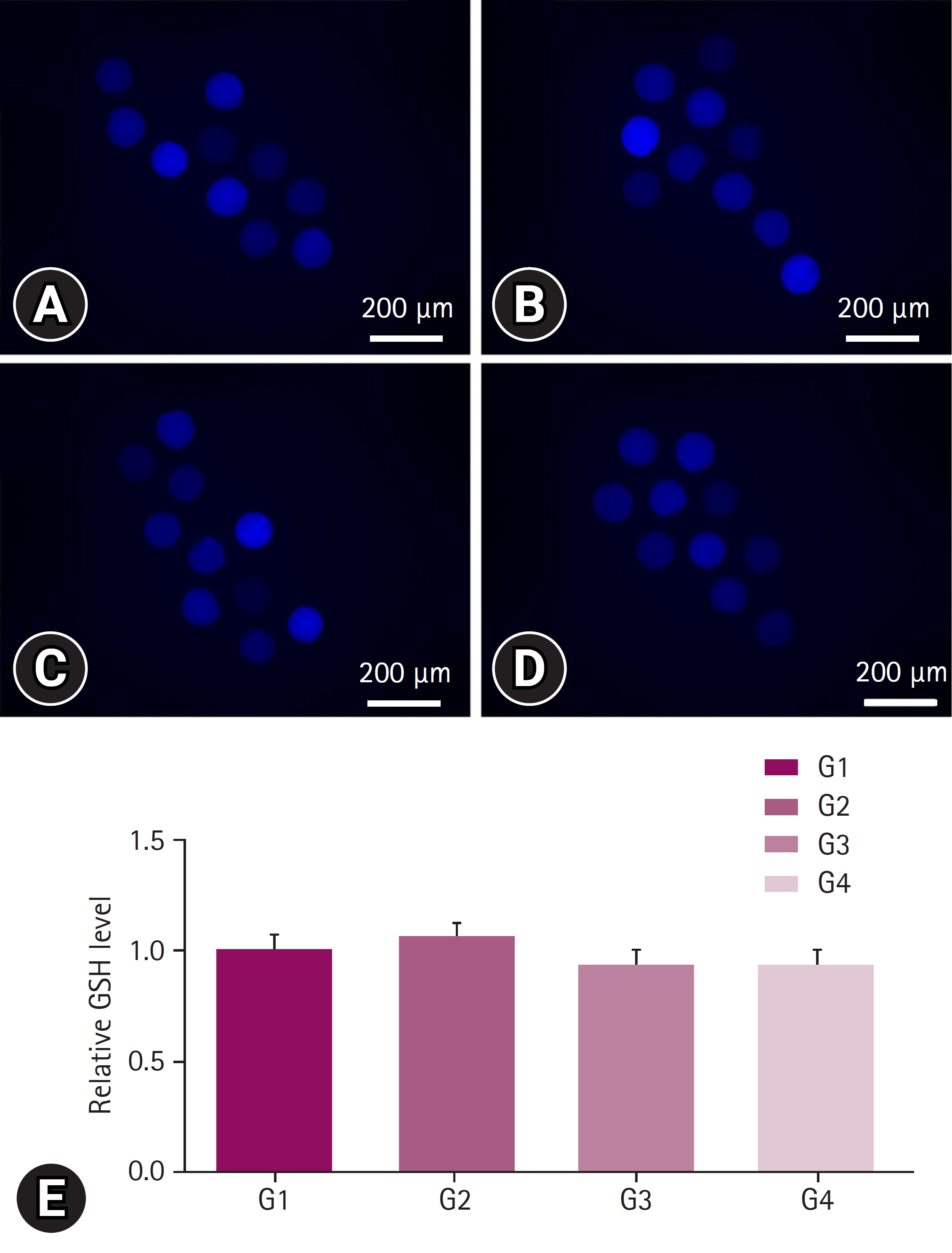
Glutathione (GSH) staining for oxidative stress measurement. The staining was performed on oocytes after 40 to 44 hours of in vitro maturation, at the stage when the first polar body was released. Oocytes were cultured in Tyrode’s medium containing polyvinyl alcohol containing 10 µM CellTracker Blue (Invitrogen Corporation, USA) in the dark for 30 minutes. At least 3 replicates were performed, and more than 30 oocytes were used. (A) Image of porcine follicular fluid (pFF) supplementation (positive control, G1) group, (B) image of pFF w/extracellular vesicle (EV) (G2) group, (C) image of EVs group (G3), and (D) image of no pFF (negative control, G4) group. (E) The GSH levels in each group were expressed relative to the mean of the positive control. Scale bar = 200 µm. Significant differences among the 4 groups were indicated with *.
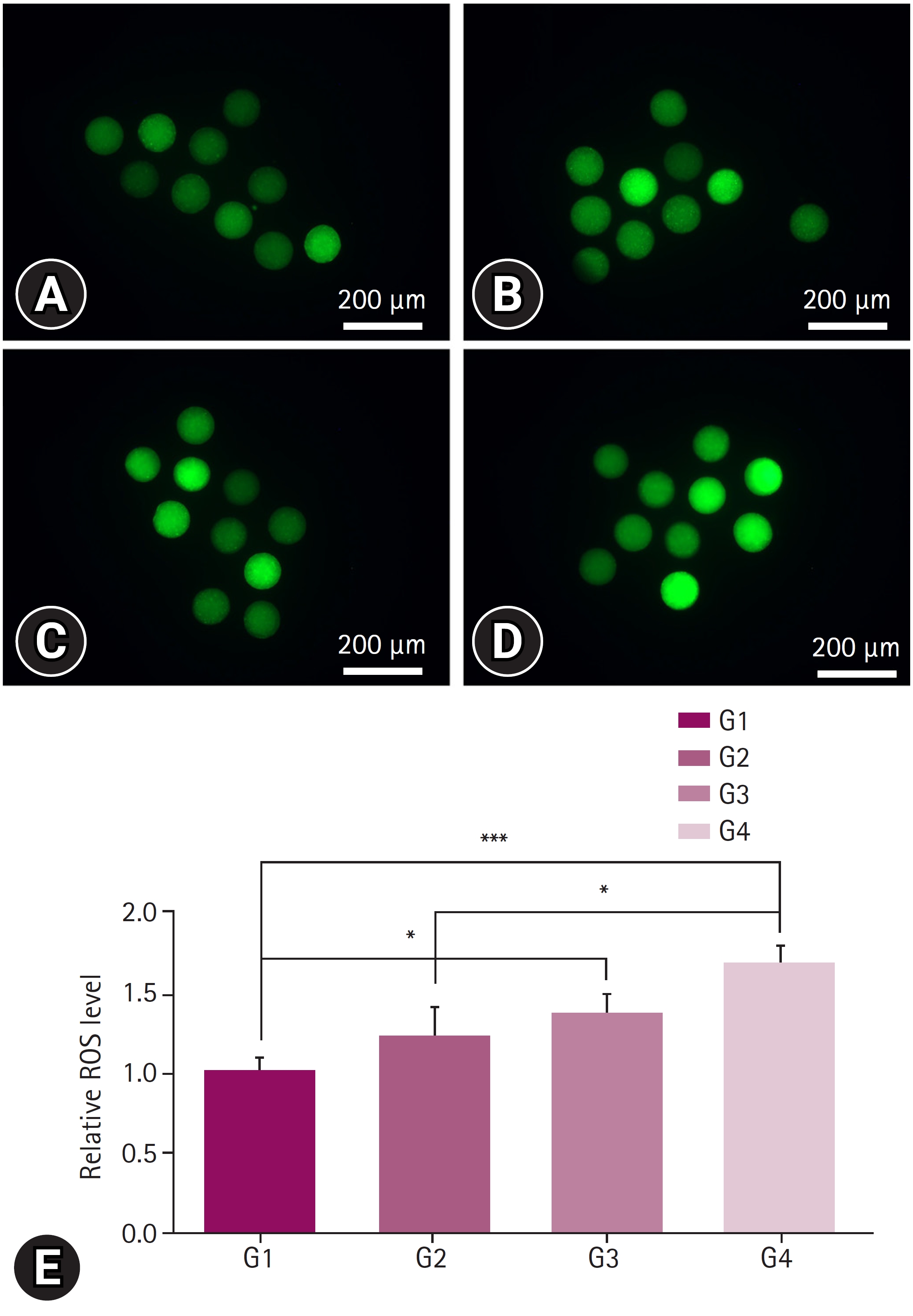
Reactive oxygen species (ROS) staining for oxidative stress measurement. The staining was performed on oocytes after 40 to 44 hours of in vitro maturation, at the stage when the first polar body was released. Oocytes were cultured in Tyrode’s medium containing polyvinyl alcohol containing 10 µM H2DCFDA in the dark for 30 minutes. At least 3 replicates were performed, and more than 30 oocytes were used. (A) Image of porcine follicular fluid (pFF) supplementation (positive control, G1) group, (B) image of pFF w/extracellular vesicle (EV) (G2) group, (C) image of EVs group (G3), and (D) image of no pFF supplementation (negative control, G4) group. (E) The ROS levels in each group were expressed relative to the mean of the positive control. Scale bar = 200 µm. Significant differences among the 4 groups were indicated with * when p < 0.05, and *** when p < 0.001.
Comparison of Embryonic development based on the presence of pFF or EVs
Cleavage rate was significantly reduced in G4 group when compared with the other 3 groups, while blastocyst formation rate was partially increased with the presence of EVs (G3) when compared with the lowest rate in G4 or with the highest rate in G1 and G2 (Table 2).
Discussion
This study aimed to investigate the effects of EVs derived from pFF on oocyte maturation. Initially, pFF was separated using ultracentrifugation, and the pellets obtained from pFF were confirmed to be EVs using NTA and cryo-TEM. Thereafter, the COCs were divided into 4 groups: pFF (positive control, G1), pFF-depleted EVs (G2), EVs (G3), and pFF was not supplemented (negative control, G4). IVM was conducted, and oocyte maturation rates were compared among the groups.
The results showed that the presence of pFF significantly affected the maturation rate of oocytes, and compared with the 2 control groups, there was no significant difference between the G2 and G3 groups. This suggests that both EVs and pFF-depleted EV may have an impact on oocyte maturation. EVs and other components in FF work together to improve oocyte maturation. The pFF contains steroid hormones, such as FSH, estrogen, and related RNA molecules, which play a role in oocyte maturation. The pFF enhances the maturation rate [18]. Some of these molecules may exist in the form of EVs and potentially contribute to oocyte maturation [13].
ROS levels as indicators of oxidative stress were the lowest in G1 and G2 groups. Increased oxidative stress has various effects on cells, including insufficient ATP production in the mitochondria, cell death, and DNA damage [19,20].
It has been speculated that these effects may also impair oocyte quality, including cleavage and blastocyst development rates [21]. Although there was no statistically significant difference between the G3 and G4 groups in terms of cleavage and blastocyst development rates, there was numerical improvement in G3 when compared with G4. Additionally, blastocyst development rates and ROS level in G3 was not significantly different from G2 or G1. This suggests that pFF-derived EVs are partially contributed to the mechanism of action of pFF and can be considered as synergistic cargo carriers of the pFF. EVs contain factors that mitigate oxidative stress and are involved in cellular maturation, contain steroid hormones, and may contribute to oocyte maturation and quality enhancement. Our results coincide with Singina et al. [22], who found a high rate of blastocyst formation after supplementing bovine oocyte with FF-derived EVs.
In summary, FF-derived EVs are partially contributing to improving the oocyte maturation and blastocyst formation.
Notes
The authors declare no conflict of interest.
Acknowledgements
This study was supported by the Research Fund of the Chungnam National University. We would like to thank Mrs. Seongja Kim and Daejeon slaughterhouse for providing pig ovaries.