 |
 |
| Korean J Vet Res > Volume 63(4); 2023 > Article |
|
Abstract
Many viruses can infect different types of birds, with poultry being the most susceptible. These viral diseases have a direct negative impact on the poultry industry, with significant economic losses. This study examined a group of the most important viruses that infect backyard chickens in 2 specific areas of Basrah Governorate, south of Iraq. The study analyzed avian influenza viruses (AIVs), Newcastle disease virus (NDV), and infectious bronchitis virus (IBV). Two hundred and ninety oropharyngeal swabs, 150 from Abu Al-Khasib and 140 from Shatt Al-Arab regions in the Basrah governorate, were obtained from backyard chickens with clear respiratory signs. The samples were subjected to viral RNA extraction, and the viral nucleic acids were detected using a reverse transcriptase polymerase chain reaction technique. The overall rate of viral infections was 74.8%, which varied depending on the type of virus: 15.8%, 31.3%, and 27.5% for AIV, NDV, and IBV, respectively. The NDV and IBV had much higher infection rates than that of AIV. In addition, the prevalence of AIV in the Shatt Al Arab district was significantly higher than in the Abul Khasib district. Moreover, there were no significant differences between the NDV and the IBV distributions in either of the targeted regions in this study.
Poultry can be infected with multiple diseases that cause considerable economic losses globally [1]. Respiratory diseases can arise from infections caused by different pathogens, including viruses, bacteria, and fungi. Among the key viral diseases that have significant importance are infectious bronchitis (IB), avian influenza (AI), and Newcastle disease (ND) [2].
AI, which is commonly known as “bird flu,” is a respiratory illness found in birds and is caused by the influenza virus type A. Although wild birds, such as ducks, shorebirds, and gulls, can carry and transmit these viruses, they may not display any visible signs of infection. On the other hand, AI has the potential to cause fatalities among domestic poultry, particularly chickens and turkeys, and occasionally in ducks and geese [3]. Avian influenza viruses (AIVs) belong to the genus Influenzavirus A and the family Orthomyxoviridae. The virus genome consists of 8 negative-sense RNA segments [4]. The AIV shows considerable variations in its external shape, such as spherical or filamentous, and sometimes it has a polymorphic or unstable shape. The genetic makeup of the virus and the host cell type play a pivotal role in determining the shape of the virus [5]. In addition, AIVs are classified into 2 distinct groups. The first includes low-pathogenic avian influenza A viruses. The pathological signs associated with infection with this type are slight or non-existent. The other type is called high pathogenic avian influenza, which is usually accompanied by typical clinical signs with high mortality rates [6]. Avian influenza is a significant concern for poultry and public health. When AIVs are present among poultry populations, cases of avian influenza in humans can be detected sporadically [7].
ND is highly contagious, spreads rapidly, and causes acute infections in domesticated birds, including chickens, turkeys, and many other bird species [8]. The disease is caused by a highly virulent strain of Paramyxovirus, which belongs to the genus Avulavirus and the family Paramyxoviridae. This virus has a non-segmented RNA genome with a negative sense [9]. Poultry is quite vulnerable to the ND virus (NDV), and many outbreaks have been documented in poultry populations worldwide. These outbreaks lead to significant financial losses, amounting to millions of dollars annually [10]. NDV strains have been categorized into 3 pathotypes based on the noticeable symptoms in chickens: lentogenic, mesogenic, and velogenic. The lentogenic form is characterized by mild symptoms; the mesogenic form exhibits moderate symptoms, and the velogenic form is highly virulent. Highly virulent strains of NDV can replicate within the central nervous system, leading to different levels of non-suppurative encephalitis and the emergence of severe neurological symptoms [11].
Similarly, IB is a highly contagious viral respiratory disease affecting chickens with acute symptoms, such as sneezing, coughing, and tracheal rales. IB can impact the kidneys, leading to reduced egg production in laying flocks [12]. The causative agent of IB is the IB virus (IBV), an RNA virus belonging to the Coronavirus genus in the Coronaviridae family. The RNA genome is represented by a non-segmented RNA genome with a positive sense [13]. Despite the availability of vaccines and their routine use in poultry production, IB is a widespread disease with significant economic consequences worldwide [14].
All 3 viruses mentioned (AIV, NDV, and IBV) play a prominent role in the occurrence of infections in backyard chickens, which can be severe. In addition, there is the potential risk of transmitting infections to humans, particularly in the case of AIVs. Limited information is available regarding the presence of these viruses in backyard chickens in Basrah province, southern Iraq. This study examined the occurrence of AIV, NDV, and IBV in this specific bird population to identify the potential for co-infection with multiple viruses.
Sample collection and all lab work were carried out according to the approved guidelines of the University of Basrah, College of Veterinary College of Veterinary Medicine, University of Basrah (approved issue number: 298 on 13 June 2022).
From October 2022 to February 2023, 290 oropharyngeal swabs were collected from backyard chickens exhibiting noticeable respiratory symptoms in 2 different regions of Basrah: 150 samples from the Abul Khasib district and 140 samples from the Shatt Al Arab district. The samples were collected from chicken houses in locations far apart to enhance the comprehensiveness of the information related to the spread of viruses in the targeted geographical area. They were placed in sterile tubes containing viral transport media, which is composed of phosphate buffer saline and glycerol (1:1). The samples were then sent to the laboratory under cold conditions. The samples were centrifuged at 1,000 × g for 10 minutes. After centrifugation, the supernatant was transferred carefully to a newly labeled tube in preparation for viral RNA extraction.
A QIAamp specialized kit for viral RNA extraction, supplied by Qiagen, was used to isolate the RNA from viruses for all samples collected, according to the manufacturer’s instructions. The extracted RNA was then quantified using a NanoDrop spectrophotometer. The extracted RNA was kept in a freezer (-20°C) until used in subsequent experiments.
The conventional reverse transcriptase polymerase chain reaction (RT-PCR) method was used to detect the presence of the 3 viruses in the study. For the AIV, a pair of universal primers was used to detect any possible type of the virus [15]. Regarding the other 2 viruses, IBV and NDV, the gene-specific primers were designed using the National Center for Biotechnology Information. These primers were designed to detect and amplify the regions of interest within the viral genomes, allowing the targeted detection and identification of IBV and NDV viruses (Table 1) [15].
One-step RT-PCR from Bioneer (Korea) was used to detect a specific region of the genome of the 3 viruses. This system relies on a single reaction to amplify the target region of the viral genome through reverse transcription (cDNA synthesis) and is then completed automatically by PCR. The starting concentration of viral RNA used (immediately after extraction) was 150 ng/μL. The RT-PCR reaction conditions were as follows: cDNA synthesis at 45°C for 30 minutes. Subsequently, the initial denaturation was carried out at 95°C for 2 minutes. Forty cycles were then performed, including denaturation at 95°C for 15 seconds, annealing at 58°C (for AIV) and 59°C (for NDV and IBV) for 40 seconds, and extension at 72°C for 40 seconds. After these steps, a final elongation period of 5 minutes at 72°C was performed. These conditions facilitated the amplification of the target regions of interest in the viral genomes. Subsequently, the reaction mixture was cooled to 4°C for 10 minutes to stabilize the reaction mixture before subsequent handling or analysis. The amplified PCR products were visualized by preparing a gel composed of 1.5% agarose in Tris-acetate-EDTA buffer and staining with Nancy-520, a fluorescent dye that binds to DNA. The PCR products were visualized under a ultraviolet transilluminator.
The data were analyzed using the IBM SPSS ver. 26.0 (IBM Corp., Korea). A chi-square (χ2) test was used to evaluate the significance of the differences between the different groups; p-values < 0.05 were considered significant, indicating a significant association among the analyzed variables.
The RT-PCR results revealed the successful amplification of genetic material from the oropharyngeal swabs collected from backyard chickens. After loading the PCR product onto a 1.5% agarose gel, distinct bands of varying sizes corresponding to the virus species were observed. The expected size of each band was determined by comparing it with a suitable DNA ladder, which served as a reference marker (Fig. 1).
This study revealed the overall proportion of infection with viral respiratory infections in backyard chickens, which was 74.8% (217/290). Among the 290 chickens examined, the prevalence of infection varied according to the type of virus. The infection rates were 15.8% (46/290), 31.3% (91/290), and 27.5% (80/290) for the AIV, NDV, and IBV, respectively. The infection rates of the NDV and IBV were significantly higher than the AIV (p < 0.5) (Table 2).
Co-infection with more than one virus was noted in a limited number of the tested birds. Only 3 samples showed positive results for the AIV and NDV simultaneously. Regarding the geographic distribution of the tested viruses, there was a significant difference in AIV between the Abul Khasib and Shatt Al Arab regions (p < 0.01). On the other hand, there was no significant difference in the prevalence of NDVs and IBVs among the studied regions. The results obtained in Abul Khasib district were as follows. Of the 150 samples tested, 14 (9.3%), 47 (31.3%), and 42 (28.0%) were positive for the AIV, NDV, and IBV, respectively. Of the 140 samples tested from Shatt Al Arab district, 31 (22.1%), 44 (31.4%), and 39 (27.8%) were positive for the AIV, NDV, and IBV, respectively (Table 3).
Many studies have provided evidence that viral respiratory infections in poultry contribute significantly to the morbidity and mortality rates and cause considerable economic losses [1,16,17]. This study investigated the most important viruses significantly impacting chicken infections, including the AIV, NDV, and IBV. These viruses were studied in 2 interesting districts in Basrah province, where animal husbandry, particularly poultry farming, is prominent. Most of the birds involved in the study showed evidence of infection with the 3 viruses tested at different rates. Hence, these viruses are widespread in the geographical provinces within Basrah Governorate. The remaining samples that were not positive for the 3 viruses included in the study may be due to the birds being infected with other pathogens, e.g., bacteria and fungi. Compared to an infection with the NDV and IBV viruses, which were similar to each other in the 2 geographical regions included in the study, the opposite was found with an infection with the AIV virus because it was higher in the Shatt Al-Arab district than Abu Al-Khasib. This difference can be explained by the Shatt Al-Arab region being the most suitable place for wild ducks because of the large number of water swamps there. This usually increases the chance of transmitting almost all subtypes of influenza A viruses.
A recent study conducted in the 2 regions in Iraq showed that the average infection with AIVs in wild ducks and broiler chickens was 72.25% and 48.45%, respectively [15]. The high prevalence observed in wild ducks could be because these birds are natural reservoirs for almost all influenza A viruses [18]. Therefore, the elevated infection rate in wild ducks may be considered normal within their natural ecological context. On the other hand, the average infection rate in the chicken population does not correlate with the findings of the current study because it was higher. This inconsistency could be attributed to the difference in chicken breeds examined between the 2 studies. This study focused on backyard chickens, while the other concentrated on broiler chickens. The variations in the infection susceptibility and immune responses among chicken breeds could be contributing factors. Different breeds of chickens may have variable levels of susceptibility or resistance to viral infections, which can affect the observed infection rates. Various factors can differ among breeds, such as immune system functionality, genetic predisposition, and overall health status, potentially leading to different infection consequences [19].
A study conducted in India to determine the prevalence of NDV in backyard chickens reported a considerably lower prevalence than that obtained from the present study. The study also showed that the prevalence of infection was significantly higher in backyard chickens than in commercial chickens [20]. Similarly, a separate study conducted in the Kurdistan region of (northern Iraq) showed a lower infection rate than the present study [21]. Numerous factors, such as the geographical region and the specific breed of birds, might influence the distribution of NDVs. Therefore, a comprehensive study encompassing a wider range of regions across Iraq and including various bird breeds would be valuable for exploring possible variations in the virus distribution. Such studies will allow any potential differences to be tracked and provide more plans for studying the dynamics of the spread and circulation of the virus.
Regarding the outcomes of IBV infection, a study conducted in 3 areas of southern Iraq revealed a higher infection rate than the present study [22]. The chicken strains in their study were commercial chickens, which differs from the present study. This discrepancy in bird species highlights the possible influence of diverse breeds on the prevalence and severity of IBV infections. Just as the findings of NDV suggest the need for future studies to explore differences in virus distribution based on geographical region and bird breeds, the results of IBV infections also indicate the importance of further investigations. Therefore, further research encompassing various bird breeds and geographical provinces will be needed to gain a more comprehensive understanding of IBV infection rates.
Overall, the prevalence of the NDV in backyard chickens in 2 districts in Basrah governorate/southern Iraq was considerably higher than that of the AIV and IBV. Regarding the geographical distribution, AIV was significantly higher in the Shatt Al Arab district than in the Abul Khasib district. In comparison, the prevalence of NDV and IBV was similar in both districts included in this study. These findings provide important insights into the relative prevalence of these viral infections in backyard chickens within specific districts in Basrah governorate.
Acknowledgments
The authors thank the College of Veterinary Medicine, University of Basrah, for its assistance in the laboratory testing.
Fig. 1.
Polymerase chain reaction (PCR) products on agarose gels stained with Nancy-520. The amplified DNA fragments from PCR were separated by electrophoresis using a 1.5% agarose gel pre-stained with Nancy-520 dye. (A) the amplified partial M gene of analyzed avian influenza virus resulted in a 108 bp DNA fragment. (B) The amplified partial F gene of Newcastle disease virus, producing a DNA fragment measuring 1,016 bp. (C) The amplified partial F gene of the infectious bronchitis virus resulted in a DNA fragment of 715 bp.
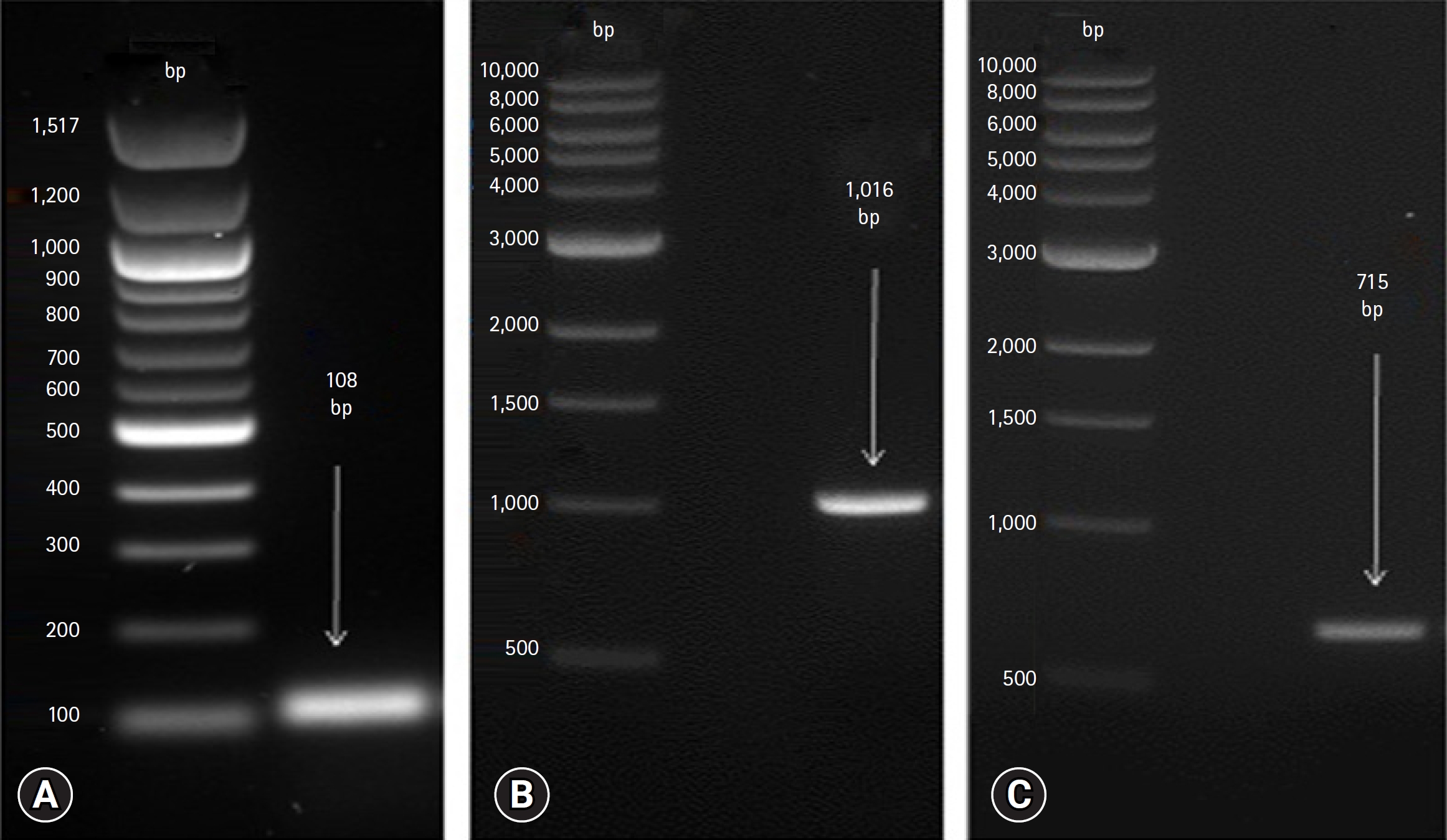
Table 1.
Primer sets used to detect the AIV, IBV, and NDV
| Virus | Primer set | Amplicon size (bp) | Reference |
|---|---|---|---|
| AIV | Forward: ATCGTCGCYTTAAATACGGT (20 bp) | 108 | [15] |
| Reverse: CGTCAACATCCACAGCAYTC (20 bp) | |||
| NDV | Forward: CAAACAGAATGCCGCCAACA (20 bp) | 1,016 | - |
| Reverse: CTGCCAACCTATCCAAGGCA (20 bp) | |||
| IBV | Forward: ACTGGTGACCAAAGCGGAAA (20 bp) | 715 | - |
| Reverse: GCTATTGCTCCGCGAAAAGG (20 bp) |
Table 2.
Total percentages of backyard chickens infected with the AIV, NDV, and IBV
| Virus | Total no. of positive samples/tested samples | Infection (%) |
|---|---|---|
| AIV* | 46/290 | 15.8 |
| NDV | 91/290 | 31.3 |
| IBV | 80/290 | 27.5 |
| Total | 217/290 | 74.8 |
Table 3.
Percentages of backyard chickens infected with the AIV, NDV, and IBV in the study areas
| Virus |
Abul Khasib |
Shatt Al Arab |
||
|---|---|---|---|---|
| No. of positive samples/tested samples | Infection (%) | No. of positive samples/tested samples | Infection (%) | |
| AIV* | 14/150 | 9.3 | 31/140 | 22.1 |
| NDV | 47/150 | 31.3 | 44/140 | 31.4 |
| IBV | 42/150 | 28.0 | 39/140 | 27.8 |
| Total | 103/150 | 68.6 | 114/140 | 81.4 |
References
1. Brown Jordan A, Gongora V, Hartley D, Oura C. A review of eight high-priority, economically important viral pathogens of poultry within the Caribbean region. Vet Sci 2018;5:14.



2. Yehia N, Salem HM, Mahmmod Y, Said D, Samir M, Mawgod SA, Sorour HK, AbdelRahman MA, Selim S, Saad AM, El-Saadony MT, El-Meihy RM, Abd El-Hack ME, El-Tarabily KA, Zanaty AM. Common viral and bacterial avian respiratory infections: an updated review. Poult Sci 2023;102:102553.



3. Dehgany-Asl S, Allymehr M, Talebi A, Yosefi O, Allahyari E. Monitoring of aquatic birds and surveillance of avian influenza and Newcastle disease of waterfowls at the National Park of Urmia Lake. Vet Med Sci 2022;8:2016-2031.



4. Bouvier NM, Palese P. The biology of influenza viruses. Vaccine 2008;26 Suppl 4(Suppl 4):D49-D53.



5. Al-Mubarak F, Daly J, Christie D, Fountain D, Dunham SP. Identification of morphological differences between avian influenza A viruses grown in chicken and duck cells. Virus Res 2015;199:9-19.


6. Rebel JM, Peeters B, Fijten H, Post J, Cornelissen J, Vervelde L. Highly pathogenic or low pathogenic avian influenza virus subtype H7N1 infection in chicken lungs: small differences in general acute responses. Vet Res 2011;42:10.



7. Chmielewski R, Swayne DE. Avian influenza: public health and food safety concerns. Annu Rev Food Sci Technol 2011;2:37-57.


8. Brown VR, Bevins SN. A review of virulent Newcastle disease viruses in the United States and the role of wild birds in viral persistence and spread. Vet Res 2017;48:68.



9. Ganar K, Das M, Sinha S, Kumar S. Newcastle disease virus: current status and our understanding. Virus Res 2014;184:71-81.



10. Amoia CF, Nnadi PA, Ezema C, Couacy-Hymann E. Epidemiology of Newcastle disease in Africa with emphasis on Côte d'Ivoire: a review. Vet World 2021;14:1727-1740.



11. Fan W, Wang Y, Wang S, Cheng Z, Guo H, Zhao X, Liu J. Virulence in Newcastle disease virus: a genotyping and molecular evolution spectrum perspective. Res Vet Sci 2017;111:49-54.


13. Quinteros JA, Noormohammadi AH, Lee SW, Browning GF, Diaz-Méndez A. Genomics and pathogenesis of the avian coronavirus infectious bronchitis virus. Aust Vet J 2022;100:496-512.



14. Toro H. Global control of infectious bronchitis requires replacing live attenuated vaccines by alternative technologies. Avian Dis 2021;65:637-642.


15. Al-Badry M, Al-Mubarak F. Molecular surveillance of avian influenza A viruses in Basrah and Wasit, Iraq. Bulg J Vet Med 2020;23:456-466.
16. Colvero LP, Villarreal LY, Torres CA, Brañdo PE. Assessing the economic burden of avian infectious bronchitis on poultry farms in Brazil. Rev Sci Tech 2015;34:993-999.


17. Mehrabadi MH, Ghalyanchilangeroudi A, Tehrani F, Hajloo SA, Bashashati M, Bahonar AR, Pourjafar H, Ansari F. Assessing the economic burden of multi-causal respiratory diseases in broiler farms in Iran. Trop Anim Health Prod 2022;54:117.


18. Hill NJ, Bishop MA, Trovão NS, Ineson KM, Schaefer AL, Puryear WB, Zhou K, Foss AD, Clark DE, MacKenzie KG, Gass JD Jr, Borkenhagen LK, Hall JS, Runstadler JA. Ecological divergence of wild birds drives avian influenza spillover and global spread. PLoS Pathog 2022;18:e1010062.



19. Aston EJ, Wang Y, Tracy KE, Gallardo RA, Lamont SJ, Zhou H. Comparison of cellular immune responses to avian influenza virus in two genetically distinct, highly inbred chicken lines. Vet Immunol Immunopathol 2021;235:110233.


20. Joshi VG, Chaudhary D, Bansal N, Singh R, Maan S, Mahajan NK, Ravishankar C, Sahoo N, Mor SK, Radzio-Basu J, Herzog CM, Kapur V, Goel P, Jindal N, Goyal SM. Prevalence of Newcastle disease virus in commercial and backyard poultry in Haryana, India. Front Vet Sci 2021;8:725232.



21. Ahmed AI, Odisho SM. Isolation identification and pathotyping of Newcastle disease viruses form naturally infected chickens in Iraqi Kurdistan Region. Iraqi J Agric Sci 2018;49:132-141.
- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 989 View
- 37 Download
- ORCID iDs
-
Firas Taha Mansour Al-Mubarak

https://orcid.org/0000-0001-5153-2283Harith Abdulla Najem

https://orcid.org/0000-0002-4203-5282Hazim Talib Thwiny

https://orcid.org/0000-0002-0047-0331 - Related articles


 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



