Pneumonia caused by Neisseria animaloris in a cat
Article information
Abstract
Neisseria (N.) animaloris is a common flora in animals, but its pathogenicity is rarely reported. In this case report, N. animaloris was isolated from a hospitalized cat with pneumonia. The cat was discharged after testing and treatment with appropriate antibiotics. This paper reports the first case of N. animaloris pneumonia in Korea.
The Neisseria (N.) genus includes 32 species of gram-negative bacteria. Most Neisseria nonpathogenic bacteria are found on the mucous membranes of animals [1]. On the other hand, several studies have reported specific examples of Neisseria causing disease in humans and animals [2,3]. Neisseria meningitidis causes typical bacterial meningitis. A case of pneumonia and bacteremia without meningitis in humans was reported [4]. Furthermore, Neisseria gonorrhoeae causes gonorrhea in humans [2], and Neisseria sp. has been associated with pneumonia in cats [1].
Neisseria animaloris is also seen as a commensal organism in the pharyngeal and respiratory tract [5]. In human medicine, infections with N. animaloris are known to occur mainly from animal bites, and one case of pneumonia caused by N. animaloris has been reported [6]. Reports of pneumonia caused by N. animaloris in dogs, cats, and humans are rare, especially in Korea. This case report describes the first case of a cat hospitalized for pneumonia caused by N. animaloris in Korea.
A 9-year-old, castrated male Abyssinian cat presented with progressive dyspnea and coughing for one month. Upon arrival at the hospital, the following parameters were measured: respiratory rate, 66/min; pulse, 128/min; body temperature, 39.6°C; blood pressure, 110 mmHg (systolic). On auscultation, a wheezing sound was confirmed throughout the left anterior lobe to the posterior lobe. A complete blood count revealed mild leukocytosis (18,480 cells/μL; reference range, 2,870–17,020 cells /μL), particularly neutrophilia (15.53×109/L). The lactate concentration (4.53 mmol/dL; reference range, 0.6–2.5 mmol/dL) and globulin concentration (6.5 g.dL; reference range, 2.5–5.1 g/dL) were high. In addition, the feline serum amyloid A level was high (59.8 μg/mL; reference range, 0–10 μg/mL). The feline serum amyloid A level is considered an indicator of the presence of inflammation [7].
Thoracic radiography revealed a round lung margin with patchy and nodular alveolar infiltration, especially in the left lobe and right caudal lobe of the lung, and left deviation of the heart (Fig. 1A and B). Lung ultrasonography revealed a shred sign.
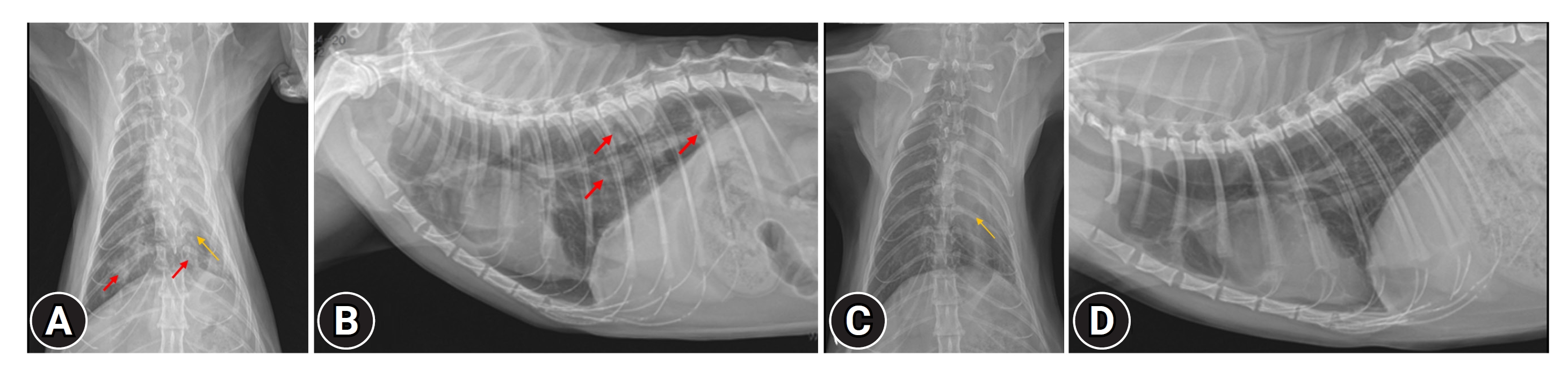
(A, B) Before treatment. (A) Thoracic radiograph (ventrodorsal view), (B) thoracic radiograph (lateral view). Patchy and alveolar infiltrations (red arrows) and left deviation of the heart (yellow arrow) were detected. (C, D) After treatment. (C) Thoracic radiograph (ventrodorsal view), (D) thoracic radiograph (lateral view). The patchy alveolar infiltration improved, but the collapsed lung did not recover (yellow arrow).
A computed tomography (CT) scan was performed under anesthesia using alfaxalone (2 mg/kg, IV, Careside, Korea) and isoflurane (2.0 %vol, Hana Pharm, Korea). A hypoattenuating lesion with rim enhancement was observed around the lung margin (Fig. 2A). Furthermore, a CT scan revealed patchy and nodular alveolar infiltration, a collapsed lobe of the left cranial lobe, and a left deviation of the heart and mediastinum (Fig. 2).
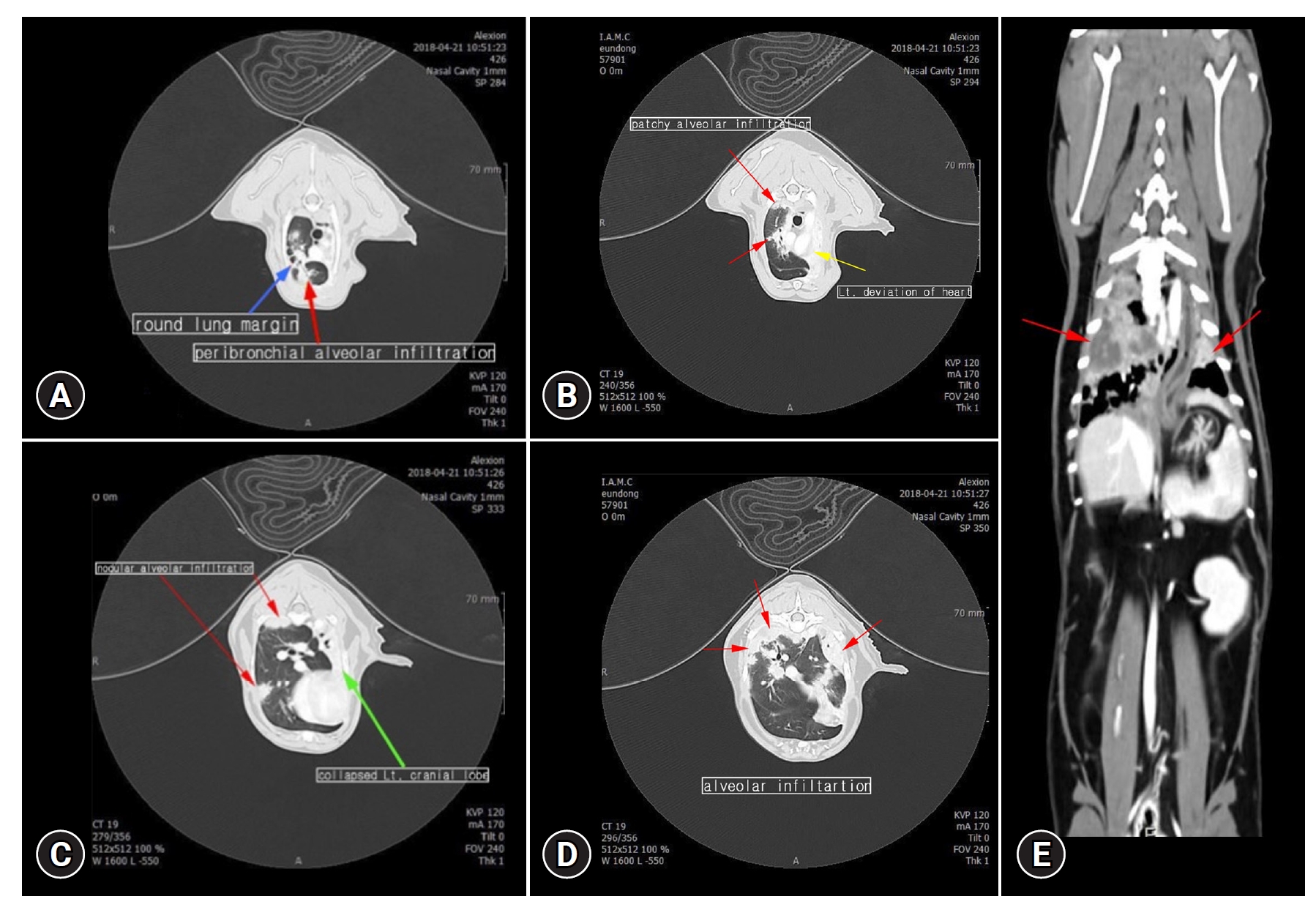
Images of computed tomography. Patchy and nodular alveolar infiltration (A–E, red arrows) with round lung margin (blue arrow), and left deviation of the heart and mediastinum (yellow arrow) with collapsed lung lobe (green arrow) were revealed.
A bronchoalveolar lavage was performed simultaneously with the CT scan. This sample was used for bacterial culture testing.
A sonograph-guided fine-needle aspiration was performed during anesthesia. The sample was obtained from the location of a right lung lobe hypoattenuating lesion with rim enhancement, and the aspiration region yielded purulent fluid. Samples were used for cytology and microbial culture.
A cytological examination showed that most of the cells were neutrophils, which mostly showed degenerative changes. In addition, macrophages, eosinophils, and red blood cells were detected, but no significant microorganisms were observed (Fig. 3).

Fine-needle aspiration of the lung lesion. Most of the cells were neutrophils. Diff-Quik stain, scale bar = 100 μm.
A bacterial and fungal culture was performed under the aerobic or anaerobic conditions. Many homologous white-colored colonies were observed in the blood agar plate after overnight culture under aerobic culture conditions. Subsequently, several colonies obtained from culture were analyzed by matrix-assisted laser desorption ionization-time of flight mass spectrometry (VITEK MS; Biomeriuex, France). The confidence score was 99.9% for the bacterial identification based on their database, indicating accurate identification of N. animaloris. The aerobic bacterial culture revealed only one type of bacteria. Subsequent polymerase chain reaction analysis revealed the bacteria to be N. animaloris. The same bacteria were identified in two samples where bacterial cultures were performed. The cultures of anaerobic bacteria and fungi were negative. This test was performed at Pobanilab (Korea).
In this case, tetracycline antibiotics (doxycycline, 5 mg/kg, twice a day, per Os [PO, by mouth]; Young Poong, Korea) and amoxicillin–clavulanate (12.5 mg/kg, Twice a day, PO [Per Os; By mouth]; Kuhnil, Korea) were administered. The antibiotic susceptibility test confirmed the sample to be sensitive to the previously prescribed antibiotic, and the prescription was maintained for four weeks.
A subsequent examination in the hospital showed that the chronic cough symptom no longer appeared. The cat’s breathing condition and auscultation sound improved significantly after treatment. In addition, respiratory rate was 30/min, indicating stable breathing. The thoracic radiograph showed that the patchy alveolar infiltration had improved. On the other hand, the collapsed lung did not recover (Fig. 1C and D).
N. animaloris is the normal flora of the oral cavity and pharynx of dogs and cats. This bacterium diplococci morphology distinguishes it from other Neisseria species, and it can grow on MacConkey agar [6].
In most cases, it is asymptomatic. On the other hand, a small number of cat cases have been reported. Recently, cases of nasofacial swelling symptoms [8] and swollen jaw signs [9] induced by N. animaloris in cats have been encountered. In another study, pyopericardium was induced by N. animaloris in cats and treated with surgical lavage and thoracic drain [10].
Few human and animal cases of pneumonia caused by bacteria belonging to the Neisseriaceae family have been reported. A case in which a person was hospitalized in an intensive care unit for pneumonia caused by N. animaloris without a history of being bitten was reported [6]. In particular, pneumonia caused by Neisseria sp. was confirmed in two cats that died of respiratory distress. The causative agent of one case revealed 99.22% and 98.56% similarity to N. animaloris and Neisseria canis, respectively, in their microbiological analysis [1]. Similarly, a human study reported that Neisseria meningitides caused pneumonia and bacteremia, unlike the usual symptoms originally caused by meningitis [4]. In addition, Neisseria flavescens can cause necrotizing pneumonia and empyema [11].
In Korea, cases of pneumonia caused by Neisseria were summarized in human medicine [12]. Most cases caused by Neisseria are human infections caused by dog or cat bites. Nevertheless, there have been cases of N. animaloris infection in dogs, cats, and humans in Korea.
N. animaloris is a common upper respiratory tract pathogen in animals. Its pathogenicity has not been studied extensively. Few strains belonging to Neisseria cause pneumonia and N. animaloris has rarely been reported to exert pathogenicity. To the best of the authors’ knowledge, this is the first report of pneumonia caused by N. animaloris in Korea.
Notes
Conflict of interest
The authors declare no conflict of interest.
Funding
This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry (IPET) through (Companion Animal Life Cycle Industry Technology Development Program), funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA), 322094-03, Korea.
Data Availability Statement
Contact the corresponding author for data availability.
Author’s Contributions
Conceptualization: Jeong SY, Cheon DS; Data curation: Jeong SY, Cheon DS; Formal analysis: Jeong SY, Park C; Funding acquisition: Park C; Investigation: Jeong SY, Park C; Methodology: all authors; Project administration: Jeong SY; Resources: Jeong SY, Park C; Software: Jeong SY; Supervision: Park C; Validation: Jeong SY; Visualization: Jeong SY; Writing–original draft: Jeong SY; Writing–review & editing: Cheon DS, Park C.
