Ebstein anomaly, right-to-left atrial septal defect, and cor triatriatum dexter in a cat: a case report
Article information
Abstract
A 6-month-old male Ragdoll cat presented with exercise intolerance. On physical examination, there was a grade 2/6 systolic murmur at the right apex. Diagnostic tests, including SpO2 measurement, blood tests, radiography, echocardiography, contrast echocardiography, and electrocardiography, were performed. Severe right atrial dilation, tricuspid valve leaflets and orifice displacement, right ventricular atrialization, septal leaflet adherence, anterior leaflet tethering, and right atrioventricular junction dilation were noted on echocardiography, alongside a right-to-left atrial septal defect. Cor triatriatum dexter and left ventricular aneurysm were observed. We diagnosed this case as having Ebstein anomaly with rare congenital heart deformities; which is rare in cats.
Ebstein anomaly is a rare congenital malformation characterized by the downward displacement of the tricuspid valve, resulting in tricuspid valve regurgitation and right ventricle (RV) atrialization [1]. Its prevalence in the general human population is approximately 0.005%, accounting for less than 1% of all congenital heart malformations [1]. In veterinary medicine, Ebstein anomaly is known to occur in 2.9% of dogs with congenital heart defects [2], whereas only a few reports exist for cats. Its clinical manifestations vary depending on factors such as severity of the malformation, age of onset, and presence of accompanying abnormalities [1]. Concomitant malformations are considered critical prognostic factors that can complicate the diagnosis and treatment of this condition [3]. Among humans with Ebstein anomaly, the most commonly observed concomitant malformations include interatrial communication (80–94%) [4], pulmonary valve stenosis, or pulmonary atresia [3], among others. In dogs, atrial septal defects (ASDs) have been found in 16% of patients [5]. However, the available data regarding concomitant malformations in Ebstein anomaly in cats is scarce. This case presents a rare occurrence of Ebstein anomaly in a cat, accompanied by right-to-left ASD and cor triatriatum dexter (CTD).
A 6-month-old, 2.8-kg male Ragdoll cat presented at Seoul Animal Heart Hospital with radiographic evidence of heart enlargement and increased N-terminal pro-B-type natriuretic peptide during pre-neutering anesthetic evaluation was asymptomatic as per the owner. Auscultation revealed a grade 2/6 systolic murmur at the right apex, and the oral mucosa was mildly pale pink; other physical examination findings were normal.
Peripheral Oxygen Saturation (SpO2), blood tests, radiography, echocardiography, contrast echocardiography, and electrocardiography were performed for further examination. SpO2 was measured at room air using a finger-adhesive sensor (Max-N; Covidien LLC, USA) connected to a Nellcor Bedside SpO2 (Covidien LLC). Measurements were acquired from the clipped proximal tail of the patient, who had an SPO2 of 92% (room air; normal: > 95%). The blood test did not reveal any significant findings.
Chest radiography (Toshiba DK-525, Toshiba E7239X; Toshiba Corp., Japan) was performed to obtain anterior and lateral images. On chest radiography, no signs of pulmonary edema or respiratory diseases were observed. Additionally, the vertebral heart score (VHS) was measured using a digital caliper on the lateral image, resulting in a VHS of 10.9v (Fig. 1).
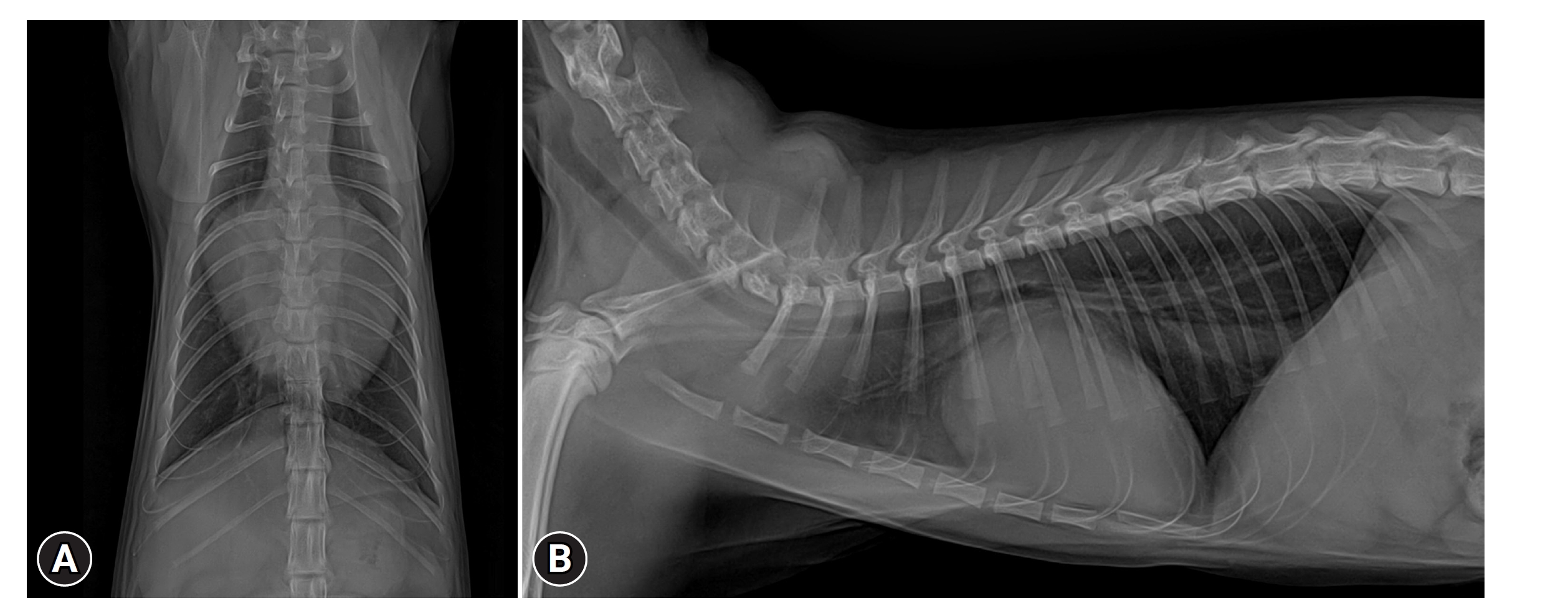
Ventrodorsal (A) and lateral (B) views of the thorax radiography showed severely enlarged heart. For review only silhouette, vertebral heart score 10.9v, with elevation of the trachea.
Echocardiography was performed using a 2.9/5.8-MHz sector transducer (GE Vivid E95 4D echo machine with R2 software; GE Medical Systems, USA) by an Asian College of Veterinary Internal Medicine-registered cardiologist. The echocardiogram revealed severe dilation of the right atrium (RA), downward displacement of the tricuspid valve leaflets and tricuspid orifice, right ventricular atrialization, adherence of the septal tricuspid valve leaflets to the septum, tethering of the anterior leaflet, right atrioventricular junction dilation, severe dilation of the right ventricular outflow tract, and pulmonary artery dilation (pulmonary trunk to aorta ratio > 1). Color Doppler showed tricuspid regurgitation (TR) and trace pulmonary regurgitation. Furthermore, the TR velocity measured with continuous wave Doppler was 2.4 m/s.
This case involved measuring representative indicators used to assess the severity of Ebstein anomaly. First, the RA diameter to left atrial diameter (RA:LA) was measured to determine the extent of RA enlargement. The diameters of the RA and left atrium (LA) were measured in the right parasternal view, resulting in an RA:LA ratio of 2.3, indicating severe transverse RA dilation (RA:LA > 2) [6]. Second, the Celermajer index, widely used to assess the severity of Ebstein anomaly in humans, was measured. In the end-diastolic left apical four-chamber view, the areas of the RA, atrialized RV, functional RV, LA, and left ventricle (LV) were measured. The ratio of the atrialized RA and RV area to the combined area of the functional RV, LA, and LV was calculated and multiplied by 100 to obtain a percentage [7]. The ratio was found to be 142%, corresponding to grade 3 in human [7].
A secundum-type ASD and a perforate CTD adhering to the right atrium's interatrial septum were noted Fig. 2). Color Doppler revealed blood flow through these membranes (Fig. 3B and C). Contrast echocardiography, using 2 mL agitated saline injected into the right cephalic vein, confirmed patency in both atria. Massive bubbles in the left heart appeared within 2 cardiac cycles post-RA opacification (Fig. 3A), leading to a right-to-left ASD diagnosis. Color Doppler showed bubble flow through the perforate CTD, similar to the observed blood flow.

(A, B) Transthoracic echocardiography performed using the right parasternal long axis 4 chamber view. The downward (apical) displacement of the tricuspid valve is shown. A part of the RV has been atrialized, and the atrioventricular junction is enlarged. Left ventricular aneurysm (*) is suspected. (C) Transthoracic echocardiography performed using the right parasternal short-axis view. Area suspected of left ventricular aneurysm (*). RV, right ventricle; RA, right atrium; LA, left atrium; LV, left ventricle, MV, mitral valve; ASD, atrial septal defect; TV, tricuspid valve.
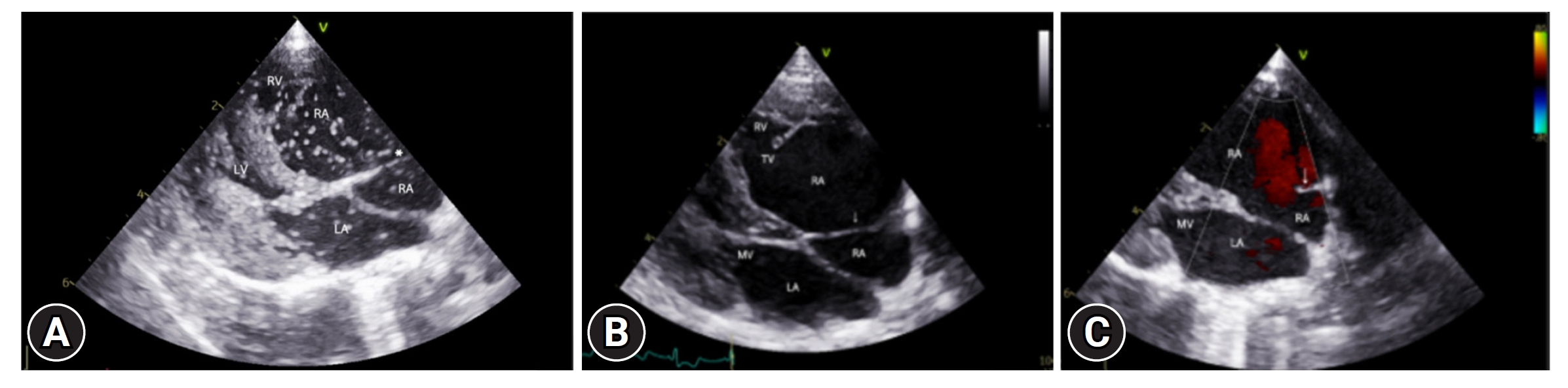
Transthoracic echocardiography performed using the right parasternal long axis 4 chamber view. (A) Membranous structure (*) separating the left atria. Massive bubbles caused by right to left atrial septal defect are observed in the LA immediately after injecting agitated saline contrast (within 2 cardiac cycles). Bubble flow through the left atrial membranous structure is also observed. (B, C) Presence of blood flow through the right atrial membranous structure. RV, right ventricle; RA, right atrium; LA, left atrium; LV, left ventricle, MV, mitral valve; TV, tricuspid valve.
The LV showed mild concentric hypertrophy, mitral regurgitation, and LV obstruction. A 0.5 cm aneurysm was suspected in the LV apical septum, which was connected to the LV with hypokinesis during ventricular contraction. Moreover, the left ventricular endocardial surface in the area suspected of aneurysm showed high echogenicity, and the interatrial septum was bowed to the left owing to right heart enlargement and elevated pressure. Hepatic vein dilation was also observed. Notably, a short P wave to R wave (PR) interval (PR 40 ms, normal 50–90 ms) was observed on the electrocardiogram. Based on these test results, the patient was diagnosed with Ebstein anomaly with right-to-left ASD and CTD.
The patient's high-severity indicators clearly indicated a severe Ebstein anomaly. Moreover, the patient had 2 rare concomitant malformations: right-to-left ASD and CTD. Given the young feline’s cyanosis and hypoxemia, combined with Ebstein anomaly, right-to-left ASD, CTD, and significant cardiac remodeling, the prognosis was deemed extremely poor. Financial constraints led the owner to decline further treatment, testing, and monitoring. Due to the absence of reported symptoms, no medications were prescribed. Authors declare human ethics approval was not needed for this study.
The patient in this case was diagnosed with Ebstein anomaly with right-to-left ASD, and CTD. Ebstein anomaly is characterized by downward displacement of the tricuspid valve, TR due to leaflet dysplasia and RV atrialization, based on echocardiography [1]. Ebstein anomaly is a rare congenital heart condition that accounts for 2.9% of congenital cardiac malformations in dogs and cats [2]. Ebstein anomaly has been extensively reported on in dogs [5]; in cats, only very brief descriptions have been observed, and there are no reports available with detailed ultrasound diagnosis, which is included in this case [8]. The clinical impact of Ebstein anomaly, often coexisting with other congenital cardiac diseases, varies based on severity and associated malformations [3]. The most common concurrent cardiac malformation is interatrial communication (ASD, patent foramen ovale [PFO]), accounting for 80% to 94% [4] of human patients with Ebstein anomaly. The occurrence of interatrial communication in dogs with Ebstein anomaly is 16% [5]. CTD is a rare congenital malformation, even in human patients [9]. Thus far, no cases of canine Ebstein patients with CTD have been reported. Therefore, this report describes a cat with 3 rare congenital malformations: Ebstein anomaly, ASD, and CTD.
Several features in this case support the diagnosis of Ebstein anomaly (Figs. 2, 3). First, the patient clearly showed that: (1) a downward (apical) displacement of the tricuspid valve and functional annulus; (2) a part of the left ventricular structure was left atrialized; and (3) the atrioventricular junction was enlarged with thinner walls. TR occurred as a result of (4) adherence of the septal leaflet to the septum myocardium and (5) tethering of the anterior leaflet [1]. These echocardiographic findings meet the diagnostic criteria for Ebstein anomaly.
Ebstein anomaly often experiences elevated right atrial pressure from birth, leading to a relatively common occurrence of ASD or PFO, accounting for about 80% to 94% [4] cases, which is very high considering the very low incidence of ASD. Canine Ebstein with ASD is commonly observed despite uncertain incidence rates. Ostium secundum constitutes 80% of human ASD cases [10], a pattern mirrored in canines. Although the possibility of an enlarged PFO cannot be entirely ruled out, considering the patient’s significant shunt size, the likelihood appears to be low. This case was also considered to have ostium secundum, similar to that seen in humans.
In Ebstein anomaly, other concomitant malformations excluding ASD are mostly rare, and their incidence is similar. Among minor congenital anomalies like pulmonary atresia, hypoplastic pulmonary artery, bicuspid or atretic aortic valves, ventricular septal defects, pulmonary stenosis, and CTD [3], CTD’s incidence is notably low [11]. This case’s pathological correlation between CTD and Ebstein anomaly persists in humans [11]. Ebstein patients with CTD generally have a worse prognosis than do those with other malformations [11], and the extent of stenosis in the membrane of CTD is critical. In this case, CTD was confirmed; however, it exhibited a nearly laminar flow owing to the large aperture.
Arrhythmias are prevalent in Ebstein anomaly cases in humans, notably preexcitation from the accessory pathway, tall P waves, supraventricular tachyarrhythmias, atrial fibrillation, or flutter [1,3,9]. Comparable arrhythmic manifestations, such as atrial fibrillation and ventricular premature beats, are observed in canine Ebstein patients [5]. In human cases, preexcitation, a typical ventricular arrhythmia cause impacting prognosis, is associated with atrioventricular developmental abnormalities [3]. In this case, electrocardiogram revealed a short PR interval (PR 40 ms, normal 50–90 ms), no delta wave, normal QRS, and T wave, which is compatible with preexcitation (Supplementary Fig. 1). Although a delta wave was not apparent, its absence is not a decisive factor in diagnosing preexcitation, as some patients with preexcitation may not exhibit a delta wave [12]. Without a delta wave, other arrhythmias must be distinguished in preexcitation cases, typically requiring electrophysiology studies, which were not conducted here. The absence of syncope history suggests a lower risk of secondary ventricular arrhythmias associated with preexcitation, despite not performing a Holter monitor test.
Ebstein anomaly is marked by the apical displacement of the tricuspid valve in the RV, due to delamination failure, which impedes the separation of the endocardium from the myocardium. This results in the downward pull of the tricuspid annulus [1]. This condition differs from tricuspid valve dysplasia, identified by valve malformation, thickening, and fusion [1].
Besides 3 major cardiac anomalies in the patient, an LV aneurysm was also noted. This is rare in the context of Ebstein anomaly, especially in humans and cats. In humans, LV aneurysms typically stem from myocardial infarction, often due to vascular or muscular diseases [13]. While myocardial infarction-induced LV aneurysms are frequent in humans [13], they are scarcely reported in cats [14]. There are no known cases of Ebstein-related LV aneurysms in dogs or cats to our knowledge.
Echocardiograms of the case revealed several characteristics resembling human aneurysm cases, including systolic hypokinesis, ventricular systole-induced herniation toward the RV, and a high-echogenic surface in the suspected aneurysmal area located in the apical and anteroseptal wall regions, where approximately 85% of human aneurysms occur [13]. Additional investigations, such as angiography, computed tomography scans, magnetic resonance imaging, ventriculography, and tissue biopsy, are necessary to determine the cause of LV aneurysm in these patients. However, an LV aneurysm is known to be associated with circulatory diseases [13]. In this regard, confirmed heart failure and various congenital abnormalities in the patient may have contributed to circulatory failure, influencing the formation of LV aneurysm. In humans, once ventricular wall aneurysm is confirmed, it exhibits an extremely poor prognosis, with a mortality rate of 80% within 1 year [15].
Treating Ebstein anomaly in humans primarily involves surgery [3]. However, such surgical methods in small animal, particularly with multiple concurrent cardiac anomalies, are challenging due to their smaller size and the nascent state of canine surgical practice. Thus, managing this condition in dogs aims to mitigate symptoms, enhance cardiac function, and avert complications, primarily via symptomatic medical therapy. In this case, we suggested symptomatic medical therapy, deferring action until the cat exhibited heart failure symptoms. However, continuous monitoring and treatment were financially unsustainable.
The survival and prognosis of Ebstein patients differ markedly among humans and dogs. Human patients may survive beyond a decade without congestive heart failure, while some dogs live several years. Conversely, certain patients succumb rapidly to sudden cardiac causes [13]. Prognosis correlates closely with various factors: significant clinical symptoms, congestive heart failure, severity level, accompanying malformations, and age at disease onset [3]. The patient presented with low oxygen saturation and cyanosis. Among several severity indicators, the Celermajer index and RA:LA ratio > 2 are known to have a close association with the survival period of Ebstein patients [5], and both of these values were found to be high in the patient. Additionally, the patient was found to have a prominent right-to-left ASD and CTD. Based on the presence of these features, particularly in conjunction with cyanosis and hypoxemia observed in a 5-month-old cat, a poor prognosis was anticipated.
This study faced limitations due to financial constraints, preventing further treatment and follow-up for the patient. Additionally, comprehensive examinations for detailed assessment were unfeasible. Despite the absence of simultaneous intracardiac ECG/pressures, echocardiography is pivotal for diagnosing Ebstein anomaly and cardiac malformations. Conducting further tests under anesthesia is particularly challenging in suspected severe malformation cases.
In conclusion, this report details an echocardiographic diagnosis of a cat with Ebstein anomaly, right-to-left ASD, and CTD, marking the first known instance of these 3 rare congenital malformations in a single patient.
Notes
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
The authors thank the cat's guardian for giving consent to publish the details of the case. Authors declare no off-label use of antimicrobials.
Funding
None.
Data Availability Statement
Contact the corresponding author for data availability.
Author’s Contributions
Conceptualization: Lee S; Data curation: Park S; Formal analysis: Park S; Investigation: Park S; Methodology: Lee S; Project administration: Lee S; Resources: Lee S; Software: Park S, Lee D; Supervision: Lee S; Validation: Park S, Oh W; Visualization: Park S, Lee D; Writing-original draft: Lee S, Park S, Lee D; Writing-review & editing: SL, Park S, Lee D.
Supplementary Materials
Supplementary data are available at https://doi.org/10.14405/kjvr.20240002.
ECG. Sinus rhythm with short PR intervals (< 50 ms).
