Evaluation of serum immunoglobulin G4 concentrations in canine pancreatitis
Article information
Abstract
The goal of this study was to measure immunoglobulin G4 (IgG4) concentrations and to evaluate the significance of these values in the management of canine pancreatitis. The medical records of 24 dogs that visited the Veterinary Medical Teaching Hospital between December 2016 and June 2018 were retrospectively reviewed to identify dogs that had been diagnosed with pancreatitis. The serum C-reactive protein and serum IgG4 concentration in the affected dogs were highly increased compared to the healthy group. Particularly, serum IgG4 measured significantly higher in dogs with pancreatitis and concurrent immune-mediated disease (p < 0.05). In conclusion, increased serum IgG4 concentrations are a characteristic finding in dogs with pancreatitis. The results of this research indicate that an elevation in IgG4 has the potential of being used as a tool for the diagnosis of pancreatitis and concurrent immune-mediated disease.
Introduction
Canine pancreatitis is a relatively common disorder that can result in a wide variety of clinical signs, and in severe cases, multiple organ failure. The mortality rate among dogs with pancreatitis remains high, ranging from 27% to 58% [1,2]. Typical clinical signs of canine pancreatitis include anorexia, vomiting, nausea, abdominal pain, abdominal distension, melena, and hematochezia.
A definitive diagnosis of canine pancreatitis requires histopathologic confirmation, but because of the invasiveness of obtaining pancreatic biopsies, and the possible existence of highly localized disease that may be missed with a single biopsy [3], this procedure is performed infrequently. Controversy exists regarding the sensitivity and specificity of diagnostic tests used for the diagnosis of pancreatitis. There is no diagnostic gold standard for canine pancreatitis. Thus, the diagnosis is often made using a combination of clinical signs, physical examination findings, elevated serum amylase and lipase values, elevated pancreatic-specific enzyme activities, increased canine pancreatic lipase immunoreactivity, ultrasonographic and computerized tomographic findings, gross lesions, and histopathologic results [4-6].
In human medicine, the classification of auto-immune pancreatitis exists [7], as well as proposed criteria for its diagnosis [8]. It is important to distinguish between pancreatitis and auto-immune pancreatitis in order to formulate a therapeutic strategy for its management in human medicine [9,10].
Over the past two decades, studies have defined auto-immune pancreatitis in humans as an immune-mediated inflammatory process [11,12]. According to the Japanese Pancreas Society and the Korean Biliary Tract Pancreas Society, elevated serum levels of immunoglobulin G4 (IgG4) are a biochemical hallmark of auto-immune pancreatitis, and therefore, diagnostic for the disease [13-16].
In recent veterinary studies, the distinctive clinical and histological appearance of canine chronic pancreatitis was found to be similar to human auto-immune pancreatitis [3,17-19]. Chronic pancreatitis in English Cocker Spaniels shows a predominance of IgG4+ plasma cells in sections of the pancreas and kidneys [20]. This suggests that the IgG4 response may prove to be a useful marker for the diagnosis of pancreatitis and concurrent immune-mediated disease.
In veterinary medicine, it is well known that the levels of IgG subtypes are higher in sick dogs than in healthy dogs [21]. Until recently, there was no report of the relationship between canine pancreatitis and serum IgG4 levels. In this study, we aimed to measure serum IgG4 levels in cases with canine pancreatitis, and to evaluate the value of IgG4 in the diagnosis of pancreatitis in dog with immune-mediated disease.
Materials and Methods
Study animals
The medical records of client owned dogs that visited Chonnam National University Veterinary Medical Teaching Hospital (CNVMTH) (Gwangju, Korea) between December 2016 and June 2018 were retrospectively reviewed to identify twenty-four dogs that had been diagnosed with pancreatitis. The pancreatitis group included dogs whose initial clinical signs included abdominal pain, vomiting, anorexia, diarrhea, melena, and/or hematochezia. Dogs from this group were selected for the study if they also possessed at least one of the following three criteria: elevated serum lipase or amylase activities; increased pancreas-specific enzyme activity, such as an abnormal SNAP canine pancreatic lipase immunoreactivity assay (cPL) (SNAP® cPL Test; IDEXX, USA); ultrasonographic (ProSound Alpha 7; Hitachi Aloka Corporation, USA) evidence of pancreatitis, such as an enlarged and hypoechoic pancreas, pancreatic edema, or hyperechoic peripancreatic fat.
The healthy group included five healthy beagle dogs. There were no remarkable findings in clinical signs and blood screening tests with SNAP cPL kit.
Epidemiologic characteristics of the all dogs such as age, breed, and sex were recorded. Concurrent diseases were categorized according to the organ system involved including endocrine, hepatobiliary, renal, hematopoietic, cardiac, respiratory, and gastrointestinal disease. Any other significant abnormalities were recorded in the concurrent disease column as well.
Clinical laboratory test results
A complete blood cell count (Procyte® DX Hematology analyzer; IDEXX) and serum biochemistry profile (Catalyst One chemistry analyzer; IDEXX) were performed to evaluate the systemic health of the dogs. A commercially available kit for measuring canine pancreatic lipase immunoreactivity (SNAP® cPL Test) was used to screen for the presence of pancreatitis. A c-reactive protein (CRP) assay (LifeAssays® Canine CRP test kit and VetReader; LifeAssays, Sweden) was also performed to look for evidence of inflammation.
Measurement of serum IgG4 concentrations
Blood samples were collected in heparin rinsed syringes, stored in Ethylenediaminetetraacetic acid (EDTA)-coated tubes, and centrifuged at 200×g for 15 minutes at 4℃. Serum samples were diluted 1:2 in phosphate-buffered saline (PBS) according to the protocol provided by the enzyme-linked immunosorbent assay (ELISA) kit manufacturer (MyBioSource, USA). Serum or standards (100 nL of each) were added to the ELISA kits, which employed the competitive enzyme immunoassay technique utilizing a polyclonal anti-IgG4 antibody and an IgG4-Horseradish Peroxidase (HRP) conjugate. The assay sample and buffer were incubated together with the IgG4-HRP conjugate on a pre-coated plate for 1 hour at 37℃. The product of any non-specific binding was washed away, and substrates A and B were added to the wells. The absorbance was measured at 450 nm. The absorbance and concentration of canine IgG4 were found to be positively correlated. The IgG4 concentration in each sample was then interpolated from this standard curve.
Statistical analyses
Statistical tests were performed using commercially available software (IBM SPSS Statistics version 23.0; IBM Corp., USA). All values were expressed as the mean ± standard deviation (SD). The Welch two sample t-test was performed to assess unequal variance in the two groups. The Pearson’s product-moment correlation test was performed to identify an association between the two variables. The chi-squared test was performed to assess two categorical variables. For all analyses, statistical significance was determined at a value of p < 0.05.
Results
Animals
Twenty-four dogs were diagnosed with pancreatitis during the study period. These were represented by the following 8 breeds: Yorkshire Terrier (n = 8); Shih Tzu (n = 6); Miniature Schnauzer (n = 3); Maltese (n = 2); and one each of Beagle, Miniature Pinscher, and Pomeranian. Fourteen dogs (58.3%) were male, and ten dogs (41.7%) were female. The mean age of the dogs was 12.5 ± 2.86 years (range, 6 to 17 years). There were no statistically significant differences between the survival and non-survival groups regarding breed, body weight, sex, or age. Selected characteristics of the groups are shown in Table 1.
Five beagle dogs were healthy group. The mean age of the beagles was 3.6 years. All beagle dogs were intact male.
Concurrent diseases that were identified in the dogs with pancreatitis are listed in Table 2. The dogs were affected by disease in at least one of the following categories: endocrine (n = 16, 66.7%), renal (n = 8, 33.3%), oncologic (n = 7, 29.2%), urogenital (n = 6, 25.0%), cardiovascular (n = 5, 20.8%), hepatobiliary (n= 4, 16.7%), immune-mediated (n= 4, 16.7%, [n = 3, immune mediated hemolytic anemia; n = 1, immune mediated thrombocytopenia]), and neurologic (n = 3, 12.5%). In addition, there were isolated cases of a thrombus, dermatitis, postoperative complications, ricin intoxication, and gastrointestinal foreign body. Only one dog in the study had no concurrent disease.
Analysis of serum CRP levels
The CRP in this study was elevated in all 24 dogs with pancreatitis. Mean levels of serum CRP in canine pancreatitis and healthy groups were 65.2 ± 53.44 mg/L (range, 20 to 210) and 5.0 ± 0 mg/L respectively.
Serum IgG4 concentrations in the pancreatitis group and the healthy group
To assess the value of serum IgG4 concentration in identifying dogs with pancreatitis, we evaluated this concentration in healthy dogs and in dogs with pancreatitis. Serum IgG4 concentration was evaluated in all 24 dogs diagnosed with pancreatitis and in 5 healthy dogs. Serum IgG4 with a cut-off value ranging from 0 mg/mL to 10 mg/mL was detected using an ELISA assay. The range and mean ± SD of IgG4 concentrations in serum samples from dogs in the pancreatitis and healthy groups are displayed in Fig. 1. The mean serum IgG4 concentrations in the canine pancreatitis and healthy groups were 2.89 ± 3.52 mg/mL (range, 0.38 to 10) and 0.90 ± 0.40 mg/mL (range, 0.43 to 1.43), respectively. Dogs in the pancreatitis group had serum IgG4 concentrations that were significantly higher than those in the healthy group (Fig. 1).

Serum immunoglobulin G4 concentrations in the canine pancreatitis group and the healthy group. Data are presented as mean ± standard deviation values of two groups. The mean serum immunoglobulin G4 (IgG4) concentrations in the canine pancreatitis and healthy groups were 2.89 ± 3.52 mg/mL (range, 0.38 to 10) and 0.90 ± 0.40 mg/mL (range, 0.43 to 1.43), respectively.
Analysis of recurrent pancreatitis in the elevated IgG4 group
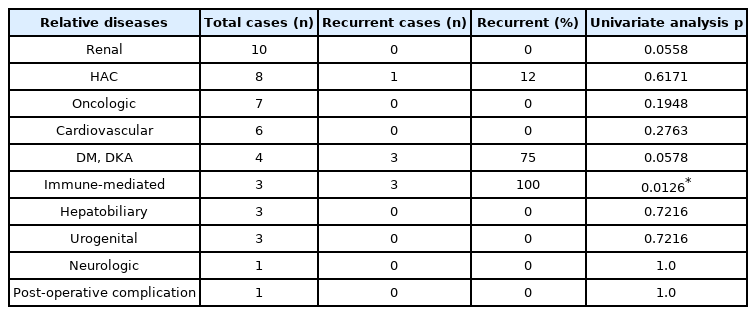
To analyze the correlation between relative factors and the risk of recurrent pancreatitis in dogs with elevated IgG4 concentrations, the relative diseases for each dog were reviewed (Table 3). In 20 of the 24 dogs with pancreatitis, the serum IgG4 concentrations were elevated. The serum IgG4 concentrations measured within the normal range in only 4 of the affected dogs. In Table 3, total cases indicates the 20 dogs with elevated IgG4 concentrations. Of these 20 dogs, 6 were cases with recurrent pancreatitis. The following concurrent diseases were represented in this study, with each of the dogs with recurrent pancreatitis being affected by at least one: hyperadrenocorticism (n = 1); diabetic mellitus (DM) and diabetic ketoacidosis (DKA) (n = 3), and immune-mediated disease (n = 3). The recurrence rate of pancreatitis in the dogs with DM and DKA in this study was 75.0% (3/4). The recurrence rate of pancreatitis in the dogs with immune-mediated disease was 100% (3/3). Among dogs with elevated serum IgG4 concentrations, there was no significant difference in risk of recurrence among those with the following concurrent diseases: renal (p > 0.05), hyperadrenocorticism (p > 0.05), oncologic (p > 0.05), cardiovascular (p > 0.05), DM and DKA (p > 0.05), hepatobiliary (p > 0.05), urogenital (p > 0.05), neurologic (p > 0.05), and postoperative complications (p > 0.05). Serum IgG4 concentrations were found to be higher in dogs with pancreatitis and concurrent immune-mediated disease; a significant difference in the risk of recurrence was also found in these dogs (p < 0.05).
Discussion
Canine pancreatitis lacks a specific treatment because almost all cases are idiopathic, which contributes to a relatively high mortality rate [1]. Recent studies in humans have classified autoimmune pancreatitis as a type in which the pathogenesis involves autoimmune mechanisms [10-12,16,22]. Over the past decade, many studies have been performed evaluating the criteria and algorithms used for the diagnosis of auto-immune pancreatitis in humans; the findings have been applied worldwide by a consensus of expert opinion [10-12,16,23]. It has been validated that elevated serum IgG4 concentrations may be used for the diagnosis of auto-immune pancreatitis in humans [10].
Therefore, the objective of this study was to evaluate the value of serum IgG4 concentrations in pancreatitis in dog with underlying diseases including immune-mediated disease (autoimmune diseases), and in doing so, to determine its usefulness in determining appropriate therapeutic strategies.
This study describes the process involved in the identification of dogs with pancreatitis at the teaching animal hospital over a 19-month period. The medical records were reviewed, and the epidemiologic characteristics, clinical signs, analyses of concurrent diseases, results of laboratory testing, and ultrasonographic findings were recorded.
In this study, all 24 dogs diagnosed with pancreatitis received an abnormal result on the SNAP cPL test [24]. Pancreas-specific lipase has been assessed in the diagnosis of canine pancreatitis in many studies [25,26]. However, it is not able to differentiate acute or chronic disease process. In fact, it is obscure to diagnose pancreatitis which show acute or chronic with clinical appearance and disease progression in the clinic. The present study investigated canine pancreatitis with clinical signs and positive reaction on canine pancreas-specific lipase. This retrospective study focused on the etiology and their mechanism based on diagnosis of pancreatitis which is not noted if it is chronic or acute. It is a limitation of the study and further studies like pancreas biopsy using laparoscopy and immune-histopathology are needed.
The acute phase reactant, CRP, is currently the gold standard serum marker for predicting the severity of disease in human pancreatitis [23]. Much attention in veterinary medicine has recently been focused on the measurement of circulating CRP, in the diagnosis and prognostication of acute and chronic inflammatory conditions [27]. Serum CRP concentrations in this study were elevated in all 24 dogs with pancreatitis which is consistent with previous reports [27,28]. The results of this study are consistent with those of a previous study [3] wherein the elevation of CRP is associated with various inflammatory diseases. In human medicine, CRP is considered to be one of the most important prognostic factors for pancreatitis and is a factor for determining the severity score according to the Japanese guidelines for the management of acute pancreatitis. Thus, the serial measurement of CRP concentration needs to be included in previous studies [3,28, 29], examining prognostic factors for pancreatitis in dogs.
In human medicine, examination of patients with auto-immune pancreatitis has revealed a high incidence of hyperglobulinemia and increased serum IgG4 levels [10]. This study has shown that the mean serum IgG4 concentrations of dogs in the pancreatitis group were significantly higher than those of dogs in the healthy group. In this study, the sensitivity and specificity of serum IgG4 concentrations for the diagnosis of canine pancreatitis 83.3% and 75%, respectively.
One study in humans describes the sensitivity and specificity of elevated serum IgG4 concentrations in the diagnosis of auto-immune pancreatitis as being 76% and 93%, respectively [27]. Based on previous human studies, elevated serum IgG4 concentrations are a characteristic of auto-immune pancreatitis, which is supported by the results obtained in this study [30,31].
We found that the outcome was significantly correlated with the presence of elevated serum IgG4 concentrations in dogs with immune-mediated diseases and recurrent pancreatitis. The serum IgG4 concentration was higher in dogs with pancreatitis accompanied by immune-mediated disease. The recurrence rate of pancreatitis in dogs with immune-mediated disease was 100% in this study. Human auto-immune pancreatitis is a disease concept that was proposed in Japan, and the auto-immune process is believed to be involved in its onset [30-35].
Therefore, auto-immune pancreatitis may be an IgG4-mediated disease [15]. Immunological examinations of patients with auto-immune pancreatitis have revealed a high incidence of hyperglobulinemia and increased serum immunoglobulin G concentrations, and IgG4 levels in particular [10].
This is the first known study to evaluate serum IgG4 concentrations in dogs with pancreatitis. Our study revealed that an elevation in serum IgG4 concentration alone is not sufficient to make the diagnosis of auto-immune pancreatitis. This study has several limitations, including a limited number of cases and the lack of a histological confirmation of the diagnosis of canine auto-immune pancreatitis. In addition, other conditions, for example, concurrent diseases should separately evaluate the effects on IgG concentration. The present study had not investigated serum IgG concentration in other conditions without pancreatitis in dogs. Finally, we had not evaluated any change of serum IgG in each recurrent case.
In the future, the elevation in serum IgG4 concentrations needs to be confirmed on pancreatic histopathology and immunostaining. Further studies are needed to resolve the limitation of the study.
Notes
The authors declare no conflict of interest.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (NRF-2020R1A2C2005364).