CD3+/CD4+/CD5+/CD8+/CD21+/CD34-/CD45-/CD79a-/TCRαβ+/TCRγδ-/MHCII+ T-zone lymphoma in a dog with generalized lymphadenopathy: a case report
Article information
Abstract
Canine T-zone lymphoma (TZL) is a mature T-cell lymphoma in dogs. The diagnosis and sub-classification are impossible without biopsy or immunophenotyping by flow cytometry. An 11-year-old, spayed, female Golden Retriever presented with lymph node enlargement. Clinical examination was consistent with canine multicentric lymphoma. However, immunophenotyping revealed positive for CD3, CD4, CD5, CD8, CD21, TCRαβ, and MHCII but negative for CD34, CD45, CD79a, and TCRγδ. Histopathology revealed lymphocytes expanding to the cortex-preserving architecture and thinning of the nodal capsule, and CD3 positive but PAX-5 negative. Owing to the indolent nature of TZL, careful monitoring approach without clinical intervention was utilized.
The most common types of canine lymphomas are aggressive and high-grade. However, recognizing indolent lymphoma is significant because it is associated with longer survival time. The most common histopathological subtypes of canine indolent lymphomas are T-zone lymphoma (TZL), marginal zone lymphoma, and follicular lymphoma. However, diagnoses of these lymphomas is mostly based on histopathological examination; thus, canine indolent lymphomas are probably underdiagnosed [1,2]. Recent reports demonstrated that a T-cell lymphoma with significant CD45 negative neoplastic cells could be classified as TZL [3-5]. However, reports describing clinical presentation, immunophenotype, molecular analysis, and histopathologic analysis of TZL in dogs remain scarce.
An 11-year-old, spayed female Golden Retriever presented lymphadenopathy, which was found upon physical examination (bilateral submandibular, prescapular, and popliteal lymph nodes), radiography and abdominal ultrasound (bilateral retropharyngeal, medial iliac, and jejunal lymph nodes). Fine-needle aspiration cytology of the lymph nodes and blood smear evaluation of peripheral lymphocytosis were performed to evaluate lymphoid neoplasia. A homogenous lymphoid population comprising small to intermediate lymphocytes that frequently had a unipolar cytoplasmic extension, “hand-mirror cells” were identified from the lymph nodes with a low mitotic rate. Laboratory analysis showed lymphocytosis (lymphocyte count, 11,890 cells/µL [reference interval, 1,050–5,100 cells/µL]) with mild leukocytosis (white blood cell count, 17,400 cells/µL [reference interval, 5,050–16,760 cells/µL]) in the peripheral blood. Peripheral blood lymphocytes were also small to intermediate in size (Fig. 1). These findings were consistent with that of multicentric lymphoma with possible bone marrow involvement (World Health Organization [WHO] stage Va) but further confirmatory tests were needed since the small to intermediate lymphocytes were predominant, and the dog only had subclinical manifestations.
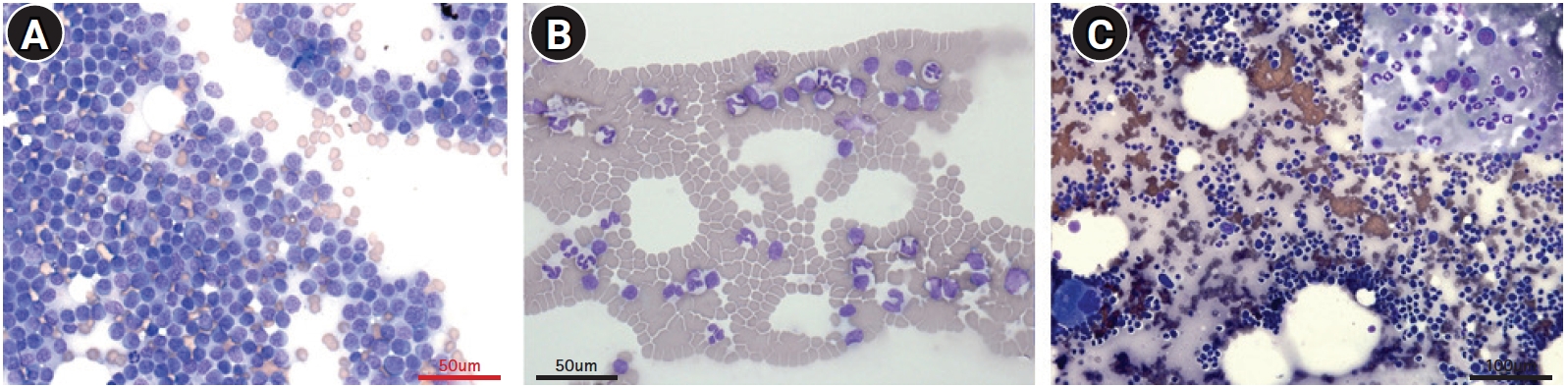
Fine-needle aspiration cytology of the lymph nodes (A), peripheral blood (B), and bone marrow (C). A homogenous lymphoid population comprising small to intermediate lymphocytes in the lymph nodes (A) with low mitotic rate. Lymphocytes from peripheral blood (B) are also small to intermediate in size. In bone marrow cytology, both myeloid and erythroid series appear complete and display an orderly maturation. The myeloid:erythroid ratio is 1.5:1, and the number of blast cell are less than 2% of the nucleated cell numbers (C). Diff-Quik stain, scale bar: (A, B) 50 μm, (C) 100 μm.
Immunophenotyping by flow cytometric analysis was performed on the lymph node aspirates and peripheral blood mononuclear cell isolates using antibodies (Table 1). Lymphocytes from the nodes and blood showed a homogenous population with both CD3+ and CD21+ but CD79a-. The CD3+ cells also showed CD4+/CD8+/CD5+/CD34-/TCRαβ+/TCRγδ-/MHCII+ but were ultimately CD45- (Fig. 2). PARR assay using the peripheral lymph node, which was performed by the Clinical Immunopathology Laboratory at Colorado State University via the IDEXX reference laboratory, showed clonal rearrangement of both TCR and immunoglobulin genes. The immunophenotyping results of CD3+/CD5+/CD21+/CD45- cells were consistent with the findings of TZL. An additional bone marrow examination showed orderly maturated myeloid and erythroid series, less than 2% of blast cells, and an estimated 1.5:1 ratio of myeloid to erythroid.
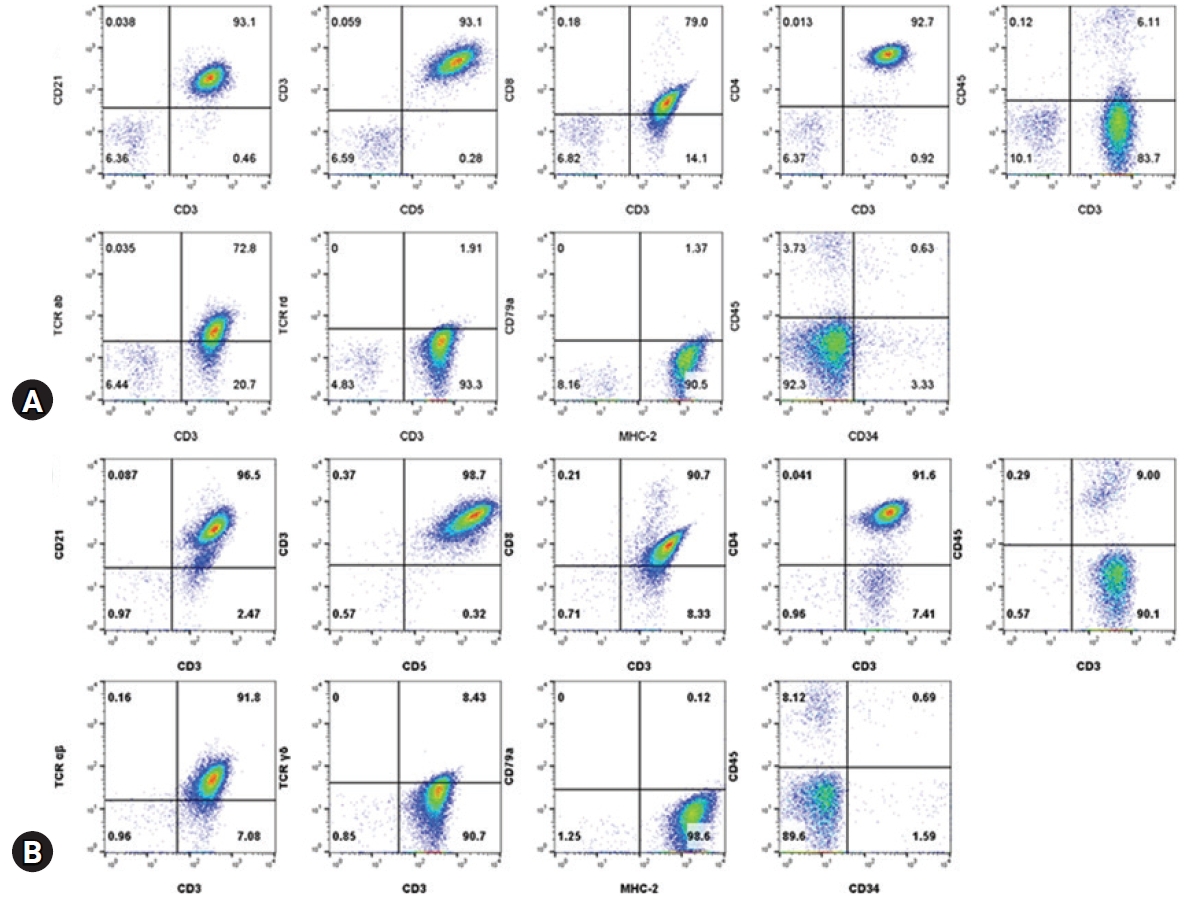
Immunophenotyping by flow cytometric analysis from the lymph node aspirates (A) and peripheral blood mononuclear cell isolates (B). Lymphocytes from the nodes and blood show a homogenous population with CD3+ and CD21+ but CD79a-. These cells also show CD4+/CD8+/CD5+/CD34-/CD45-/ TCRαβ+/TCRγδ-/MHCII+. Lymphocytes were selected by logical gating and CD14- cells.
Histopathologic examination of the right prescapular lymph node confirmed TZL based on the WHO classification of canine malignant lymphomas. Interpreted neoplastic cells, which were small to intermediate sized, expanded to the cortex and paracortex, and to a lesser extent, the medullary cords; however, overall nodal architecture was preserved as thinning of the nodal capsule and transmural invasion of the neoplastic lymphocytes were not detected. Likewise, evidence of neoplastic cell extension into the perinodal tissues was absent. The peripheral sinus was compressed due to neoplastic cell expansion with remnant fading germinal centers. Immunohistochemistry revealed positive cytoplasmic immunoreactivity for CD3 in more than 95% of the round cells; many of these were compressed against the capsule, causing the capsular sinus to collapse, and often incorporated random lymphoid follicles. There was no appreciable immunoreactivity of PAX-5 in the neoplastic round cell population (Fig. 3).
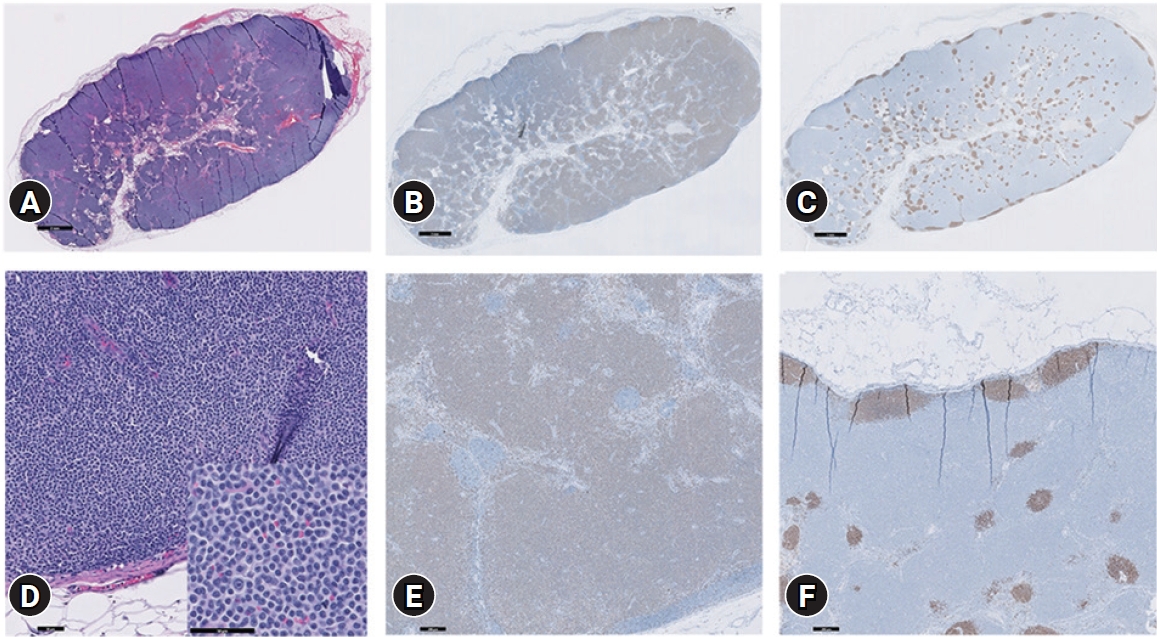
Histopathologic and immunohistochemistry examination of the right prescapular lymph node. On H&E staining (A, D), small-to intermediate-sized cells (D, inset) expanding to the cortex and paracortex can be seen. The overall nodal architecture is preserved. Thinning of the nodal capsule and transmural invasion of the neoplastic lymphocytes are not detected. Evidence of neoplastic cell extension into the perinodal tissues is also absent. The peripheral sinus is compressed due to neoplastic cell expansion, with remnant fading germinal centers. Immunohistochemistry reveals positive cytoplasmic immunoreactivity for CD3 is greater than 95% of the round cells, and many of these are compressed against the capsule, collapsing the capsular sinus, and often incorporate random, original/remnant lymphoid follicles (B, E). There is no appreciable immunoreactivity with PAX-5 in the neoplastic round cell population (C, F). Scale bar: (A-C) 2 mm, (D) 50 μm, (E, F) 200 μm.
Owing to the clinically indolent behavior and limited response to chemotherapy of TZL, the dog did not receive any clinical intervention; careful monitoring was recommended after discussion with the owner.
Canine TZL is categorized as an indolent lymphoma. Golden Retrievers and Shih-Tzus have been overpresented in TZL [3]. It is associated with long-term survival without clinical signs and rarely requires clinical intervention. Although TZL is frequently found in dogs [3], diagnosis without immunophenotyping or surgical resection was challenging in this case.
First, the clinical signs were vague. Previous studies revealed that almost all canines with TZL present with generalized lymphadenopathy without clinical signs of illness which can last for a year [6-8]. Hepato/splenomegaly, cutaneous lesions, pale mucous membranes, and lethargy can be observed but are non-specific. Generalized lymphadenopathy was the only reason for referral in this case, and the dog was asymptomatic.
Second, peripheral lymphocytosis (lymphocyte count, 11,890 cells/μL) was the only abnormal laboratory finding in this case. Moreover, lymphoproliferative disease could not be confirmed without peripheral blood immunophenotyping because malignant cellular changes were not found in the blood smear examination. As cytologic evaluation of the bone marrow revealed mature lymphocytosis, orderly maturation, and bone marrow examination might not clarify the peripheral lymphoproliferative disorder. Bone marrow involvement of neoplastic cells (WHO lymphoma stage V) is uncommon in previous reports [1,3,7]. This can be explained as an overspill phenomenon; neoplastic cells in lymph nodes are released into the peripheral blood, rather than neoplastic invasion into the bone marrow [9]. Furthermore, negative for CD34 indicates leukemic stage was less likely.
Third, cytologic evaluation of the lymph nodes showed a highly prevalent population of small to intermediate sized lymphocytes, which did not allow definitive determination of reactive hyperplasia or low-grade lymphoma. Moreover, reactive paracortical hyperplasia cannot be completely ruled out based on cytological evaluation alone [5]. Thus, histopathological examination should always be performed; accuracy without immunohistochemical phenotyping was 80% for classified indolent lymphoma [5].
Lastly, clonal rearrangement was seen in both immunoglobulin and TCR genes. Although the specificity is generally high, the sensitivity of PARR is low; thus, molecular diagnosis for clonality is not reliable despite its invasiveness. Among eight cases of TZL, clonal rearrangement was detected in five dogs [8]. Moreover, only one of the five dogs with clonal rearrangement showed dual positivity for immunoglobulin and TCR genes. In previous reports, in immature B-cell neoplasia, such as precursor B-acute lymphoblastic lymphoma or acute myeloid leukemias and mature B-cell malignancies, cross-lineage TCR gene frequently occur because containing TCR gene rearrangements. Likewise, cross-lineage immunoglobulin gene arrangement occur in T-cell malignancies, but lower frequency [10]. Further studies on molecular typing of TZL should be performed in the future.
Fortunately, previous studies have shown that immunophenotyping is an important tool for the classification of TZL. Results from both the lymph nodes and peripheral blood were CD3+/CD4+/CD5+/CD8+/CD21+/CD34-/CD45-/CD79a-/TCRαβ+/TCRγδ-/MHCII+ in this case. The characteristic phenotypic features of TZL are CD45-/CD3+/CD5+ and high levels of expression of CD21 and class II MHC [5,7,11]. CD45 is a prototypic receptor-like protein tyrosine phosphatase, which has multiple isoforms that are expressed by alternative splicing of the CD45 extracellular domain. Two major isoforms are found in canine T-lymphocytes, and their functions modulate the immune system by regulating signal transduction. CD45 is an obligate positive regulator of antigen TCR signaling. Perturbation of CD45 function is associated with immunological and nutritional disturbances as well as hematologic malignancies such as multiple myeloma [12]. In TZL, loss of CD45 expression may contribute to neoplastic transformation of T-zone lymphocytes [5,7]. One possible explanation is that the loss of CD45 expression may allow T-cells to escape destruction in the thymus or to evade apoptotic signals in the periphery, leading to eventual neoplastic transformation [13]. CD3+ lymphocytes also showed CD4+, CD8+, and CD21+ expression in this case. Co-expression or loss of CD4 and CD8 can also be suggestive of lymphoid neoplasia compared to reactive nodes, but the difference in prognosis between dual positive and dual negative presentations is unknown. CD3+ cells also showed high levels of CD21, a B-cell marker. In addition, 76.9% of the TZL samples showed CD21+ expression, whereas only 15% showed CD79a+ expression according to a previous study [5]. If expression of CD21 is highly identified simultaneously with phenotypic characteristics of TZL, definitive diagnosis by histopathological examination is required to rule out of concurrent B-cell neoplasia [14,15]. In addition, most CD3+ cells are positive for TCRαβ but negative for TCRγδ. Taken together, these results suggest that CD3+ cells may arise from activated precursor T cells [7]. Immunophenotyping identical to that of our case was only seen in one out of total 26 TZL cases in another study [5]; clinical implication of the other antigen expression should be studied in the future. In cases of double-positive for B-/T- cell population in flow cytometric analysis, immunohistochemistry is essential. This diagnostic limitation of flow cytometry is due to lab errors from delayed process between sampling and analysis which can result in loss of expression and intensity of cell markers.
In situations where an indolent lymphoma is suspected, the analysis of expression of CD45 through flow cytometry can be used as a decisive diagnostic tool for TZL. Owing to its non-invasive approach, immunophenotyping using flow cytometry is a useful diagnostic tool for the tentative diagnosis of TZL.
Notes
The authors declare no conflict of interest.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2020R1C1C1008675).

