Cutaneous angiomatosis in a dog: a case report
Article information
Abstract
A 1-year-old castrated mixed-breed dog presented with diffuse, purple lesions arranged in an irregular patchy pattern, with a slight elevation on the right hindlimb extending from the tarsus joint to the upper region of the thigh. Dermatological examinations and fungal and bacterial cultures revealed no infectious agents. The therapeutic response to antibiotics and antifungal agents was negative. A histopathology examination of the lesion revealed vascular proliferation with vasodilation and numerous varying-sized vessels. Mast-cell-dominated perivascular cuffing was also noted. The dog was diagnosed with cutaneous angiomatosis due to diffuse lesions and the histopathology findings of hemangioma.
Angiomatosis and vascular tumors, including hemangioma, hemangiosarcoma, lymphangiosarcoma, and vascular hamartoma, are characterized by endothelial cell proliferation [1]. Angiomatosis is a rare benign vascular proliferative change that can be induced by bacterial infections, the repair response to a primary injury to damaged tissue, or an unknown origin [2-4]. Angiomatosis lesions have been reported to arise in the skin, viscera, vertebrae, meninges, intestine, and ovary [3]. Cutaneous angiomatosis is a rare disease in veterinary medicine, with only seven dogs with cutaneous angiomatosis reported [2,5-9]. Surgical excision methods, such as conventional surgery, coagulation laser therapy, and radiation, are the only treatments for cutaneous angiomatosis [6-8].
To the best of the authors’ knowledge, canine cutaneous angiomatosis has never been reported in Korea. This case report describes canine cutaneous angiomatosis diagnosed based on the clinical features, dermatological findings, and histopathology findings.
A 1-year-old castrated male mixed-breed dog presented to Chungbuk National University Veterinary Teaching Hospital for gradually enlarging purple lesions on the right hindlimb that had developed 9 months prior (Fig. 1). The lesions extended from the tarsus joint to the thigh and were elevated, with an irregular patchy pattern of discoloration. The dog had no history of trauma involving the right hindlimb, and whole-body radiography revealed no fractures or other abnormalities induced by trauma. Furthermore, the lesions did not induce pain or lameness. The results of the skin cytological evaluation for infectious microorganisms and superficial and deep skin scrapings were negative. A complete blood count revealed no remarkable findings.

Right hindlimb of a dog with cutaneous angiomatosis. (A) Multiple, diffuse, red to purple, discolored lesions were observed generally on the right hindlimb. (B) The lesions were observed as an irregular patchy pattern with slight elevation.
Hyperproteinemia (7.5 g/dL; reference interval, 5.4-7.1 g/dL) and hyperalbuminemia (3.5 g/dL; reference interval, 2.6-3.3 g/dL) were observed on serum biochemical analysis; on the other hand, C-reactive protein and creatine phosphokinase levels were not increased. Despite the negative skin cytology results for microorganisms, deep pyoderma and dermatophytosis were not completely excluded because of the widespread lesions on the skin, including the dermis. Bacterial and fungal cultures were performed for diagnosis because of the possibility of a false negative skin cytology result due to an incomplete skin test. Itraconazole (Ashicona; ALS, India) 10 mg/kg once daily and amoxicillin/clavulanate (Lactamox; A Progen Pharma, Korea) 25 mg/kg twice daily were prescribed for a therapeutic diagnosis of deep pyoderma and dermatophytosis until the culture was completed. On the other hand, the skin lesions on the right hindlimb did not improve, and the bacterial and fungal cultures were negative. Therefore, infectious skin diseases were excluded, and antibiotics and antifungal agents were discontinued. Histopathology analysis was performed on punch biopsy specimens obtained from the right hindlimb lesions. After collection, the samples were fixed in a 10% phosphate-buffered formalin solution. Hematoxylin and eosin (H&E) staining was performed before optical microscopy, which revealed the proliferation of vessels with vasodilation and inflammation (Fig. 2). Diffuse vascular proliferation consisted of numerous capillaries of variable diameters in the dermis, irregular filling of blood cells and fibrin within the proliferated blood vessels, and perivascular cuffing in the upper region of the dermis. In the perivascular cuffing region, mast cells were observed mainly among the inflammatory cells, and the infiltration of plasma cells was also observed (Fig. 3). Microorganisms, such as bacteria and parasites, were not identified. Pleomorphism was not sufficient to indicate malignancy. The histological findings suggested hemangioma. On the other hand, the dog was younger than expected for hemangioma, and the lesions appeared diffuse and inconsistent with hemangiomas. Vasculitis was not ruled out because of perivascular inflammation, and 0.5 mg/kg of prednisolone (Solondo; Yuhan, Korea) twice daily for 2 weeks was prescribed. On the other hand, no clinical improvement was observed during the follow-up. Therefore, the dog was diagnosed with angiomatosis considering the presence of diffuse lesions, histology findings of blood vessel proliferation, and the absence of a response to treatment for vasculitis and infection. After that, the dog did not present to the veterinary hospital and could not receive additional treatment, including laser phototherapy. The total follow-up period was 45 days.

Optical microscopy findings of the right hindlimb lesion. (A) Vascular proliferation and vasodilation in the upper dermis (H&E, ×40); inset square represents (B). (B) The hemangioma pattern was observed in the lower part of the dermis, where vascular proliferation and vasodilation were connected in the hair follicle (H&E, ×100).
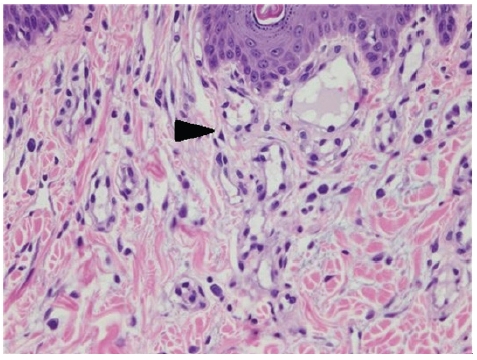
Microscopy findings of the right hindlimb lesion. Inflammatory cells were mainly mast cells (arrowhead) (H&E, ×400).
Cutaneous angiomatosis is typically characterized by variable-sized blood-filled vascular structures separated by apparently normal or myxomatous mesenchymal tissue in the dermal and subcutaneous regions [10]. Fibroblasts, pericytes, and smooth muscle cells, accompanied by proliferating endothelial cells, can be seen in some forms of angiomatosis [1]. Diseases with the characteristics of vascular proliferation, such as hamartoma, vascular hyperplasia, hemangioma, and hemangiosarcoma, should be ruled out for a diagnosis of angiomatosis [7].
The right hindlimb lesions in this dog were elevated with diffuse red to purple discoloration, similar to the gross lesion reported previously in canine cutaneous angiomatosis [2]. Cutaneous angiomatosis in humans usually occurs in the lower extremities, followed by the chest wall, abdomen, and upper extremities [11]. On the other hand, the reported dog also had lesions on the extremities [2]. This case also coincides with previous reports in that the lesions occurred in the right hindlimb.
The histology findings of the previously reported dog with cutaneous angiomatosis included multiple capillary vessels and endothelial-lined cavernous vessels scattered and infiltrated throughout the superficial and deep dermis, surrounding adnexa, and extending to the subcutaneous adipose tissue and muscle [2]. Two major histological patterns of angiomatosis can be found in humans. The first is the haphazard proliferation of varying-sized vessels and clusters of capillary vessels adjacent to the vein walls. The second is a central large vessel surrounded by clusters of capillary-sized vessels arranged in nodules [11]. In the present case, mild spongiosis was observed in the epithelium, and vascular proliferation with vasodilation of the upper dermis and inflammatory cell infiltration into the peripheral tissues were observed. Most of the infiltrated inflammatory cells were identified as mast cells, but plasma cells were also identified. Vascular proliferation with vasodilation as a histologic pattern of hemangioma was observed in the lower part of the hair follicle of the dermis. The cells lining the vessels were identified as well-differentiated endothelial cells. These histopathological findings were consistent with previous findings in humans and dogs with cutaneous angiomatosis [2,11].
The present case showed mast cell-based perivascular inflammatory cell infiltration, which was not a previously reported histology finding of angiomatosis. Several mast cell-derived mediators, including vascular endothelial growth factor, basic fibroblast growth factor, transforming growth factor-β, tumor necrosis factor-α, and interleukin-8, are angiogenic and regulate endothelial cell proliferation [12]. Furthermore, mast cells may play important roles in the development and progression of canine cutaneous hemangioma, hemangiosarcoma, mammary adenoma, and adenocarcinoma [13]. In this case, the infiltration of mast cells around the blood vessels is thought to be due to excessive vascular proliferation. On the other hand, because immune-mediated diseases, including vasculitis, could not be ruled out, an immunomodulatory dose of prednisolone was prescribed for a therapeutic diagnosis, but the lesions did not improve. Therefore, immune-mediated diseases were excluded from the differential diagnosis.
Cutaneous hemangioma has a high incidence in older dogs. The appearance of a solitary, round, well-circumscribed, firm to fluctuant, raised bluish-to-reddish black dermal or subcutaneous growth ranging from 0.5 to 4 cm in diameter has been reported [14]. In this case, vascular proliferation was identified through histopathology. On the other hand, malignancy was not observed, suggesting the possibility of diffuse hemangioma, indicating cutaneous angiomatosis [2,9,10]. Hemangiosarcoma was also excluded from the differential diagnosis, considering the appearance of the lesion and the absence of findings of malignancy on histopathology.
Although bacterial infection and trauma can cause angiomatosis, angiomatosis without specific causes has also been reported [2-4]. Bacillary angiomatosis is one of the most common types of angiomatosis caused by bacterial infections [4]. Therefore, bacillary angiomatosis was a differential diagnosis in this dog. On the other hand, bacillary angiomatosis did not correspond to this case because no infectious agents were found in the dermatologic examination or histopathology. Trauma-induced angiomatosis was also excluded based on the patient’s history, physical examination, and radiology investigation.
The results of the blood analysis were unremarkable. Hyperproteinemia with hyperalbuminemia in this dog was suspected of having resulted from dehydration. Therefore, this case was presumed to be a cutaneous angiomatosis of unknown origin.
The dog was finally diagnosed with cutaneous angiomatosis of an unknown origin based on the signalment, gross lesions, exclusion of other diseases, and histopathology results. A previously reported dog with progressive cutaneous angiomatosis presented with slow progression over approximately 10 years [7]. Because the follow-up period was also short (45 days), it was impossible to accurately determine if the cutaneous angiomatosis in this dog was progressive.
This case report has several limitations. First, the cause of cutaneous angiomatosis was not identified. A Bartonella infection was not excluded because tests to identify Bartonella, such as the Warthin-starry stain and polymerase chain reaction, were not conducted. Second, although vascular proliferation was identified by H&E staining, CD31 immunohistochemistry, a confirmatory marker of vascular proliferation, was not performed [15].
In conclusion, dogs with diffuse skin lesions and a hemangioma pattern on histopathology can be diagnosed with cutaneous angiomatosis after excluding other possible diseases, including cutaneous infectious diseases, hemangioma, hemangiosarcoma, and vasculitis. This report presents the first case of canine cutaneous angiomatosis in Korea.
Notes
The authors declare no conflict of interest.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2021R1A2C1012058).
