Supplementation of cryoprotective extender with resveratrol decreases apoptosis index and reactive oxygen species levels in post-thaw dog sperm
Article information
Abstract
Resveratrol (RSV, 3,5,4′-trihydroxytrans-stilbene) protects sperm from cryo-induced damage in various animal and human species. In this study, we aimed to assess the effect of dog sperm cryoprotective extender containing RSV on the quality of post-thaw dog sperm. Sperm were collected from 4 Beagles and supplemented with different concentrations of RSV (0, 100, 200, and 400 µM). After thawing, apoptosis index, and reactive oxygen species (ROS) levels were assessed to determine post-thaw sperm quality. Dog sperm cryopreserved with 400 µM RSV showed significant improvement in post-thaw sperm quality with lower apoptosis index and ROS levels (p < 0.05). Our results showed that the supplementation of dog sperm cryoprotective extender with RSV at a concentration of 400 µM improved the post-thaw dog sperm quality in the term of sperm ROS production and apoptosis. In addition, we emphasize the necessity of testing the ROS levels and apoptosis index using flow cytometry to determine the quality of post-thaw semen.
Introduction
The use of artificial insemination (AI) has expanded widely in livestock and canine reproduction. This technique reduces the use of breeding dogs, which requires transportation of animals, is relatively costly, is time-consuming, and poses risks of disease transmission [1-3]. AI also facilitates breeding of the required, usually genetically superior dogs, both within and among countries [4,5]. Cryopreservation of genetic materials in livestock has various limitations that negatively affect fertility quality [6-9]. Cryo-induced damage causes alteration of sperm cell homoeostasis and leads to failure of the antioxidant system, which plays a major role in controlling the level of oxidative stress (OS) and is responsible for controlling excessive free radicals [10].
Various reports have shown that supplementation of antioxidants in cryopreservation medium improves post-thaw sperm quality [4,11]. Cryopreservation efforts involving freezing and thawing inevitably affect sperm homeostasis, leading to significant physiological and chemical changes in the sperm, affecting OS homeostasis and causing excessive production of reactive oxygen species (ROS) [12,13]. This is mainly due to the removal of seminal plasma during the cryopreservation process, which reduces antioxidant defenses in sperm and makes sperm vulnerable to OS [14]. Other effects of cryopreservation include osmotic stress, cold shock, and intracellular ice crystal formation [15-17]. Various antioxidants have been shown to improve post-thaw sperm quality in different animal species by suppressing ROS production and its associated effects [18,19]. Previous studies showed that supplementation of cryoprotectant extender with kinetin and astaxanthin improved post-thaw motility and structural integrity of dog sperm [20,21]. Resveratrol (RSV, 3,5,4′-trihydroxytrans-stilbene) exhibits its antioxidant effects by scavenging free radicals, chelating divalent cations, and inhibiting ROS formation [22]. Supplementation of extender with RSV also decreased mitochondrial membrane potential and reduced ROS levels [23]. RSV supplementation in the cryopreservation of human sperm has been reported to improve the quality and function of post-thaw sperm [24].
Accordingly, we expedite the possible application of flow cytometry, for the detection of the survival rate depend on apoptosis index, which is vital for the sperm quality [25]. Besides, apoptosis elicited from cryopreservation and post-thaw effects, sperm are experiencing from relatively higher ROS [26]. During freezing, an increase in ROS level in sperm leads to fatty oxidation. This action induces DNA fragmentation in sperm, leading to apoptosis by relying on damage to the genetic mechanism [27]. Thus, we aimed to use the flow cytometry as independent test for assessing the quality of post-thaw sperm in dog sperm. We applied the apoptosis index and ROS level for the detection of the quality of sperms analysis of the control and RSV treated. In fact, even there are reports of application of flow cytometry, there are few and most of reports used as an additional method of assessment to conventional techniques. Therefore, this study aims to evaluate the effect of RSV on ROS levels and apoptosis in dog sperm, as measured by flow cytometry, independently analyze post-thaw quality.
Materials and Methods
All chemicals used in the experiment were purchased from Sigma-Aldrich (USA), unless otherwise stated. Sperm cryopreservation and post-thaw quality assessments were performed following previously reported protocols [13]. All experimental procedures were conducted according to the guidelines for the care and use of laboratory animals at Chungnam National University (approval number: 202006A-CNU-103).
Preparation of buffers
The sperm washing and dilution system was prepared as described previously [20]. Buffer 1 used for sperm washing was composed of Tris (hydroxymethyl) aminomethane (198.11 mM), citric acid (72.87 mM), fructose (44.39 mM), and kanamycin sulfate (0.25 mM) dissolved in distilled water (pH 6.6, 290 mOsm). Buffer 2 (semen extender) was prepared using 54% buffer 1 (volume per volume [v:v]), 40% egg yolk (v:v), and 6% glycerol (v:v).
Study animals and semen collection
Four healthy male Beagles, 3- to 4-year-old and weighing 8 to 12 kg, were used for semen collection. Each dog was kept under the same conditions according to standard protocols, including protection from excessive noise, and provided a standard diet with sufficient area for exercise and rest. Semen collection was performed twice a week for a total of 8 times using digital manipulation. Semen samples were initially assessed using a CASA software imaging system (MICROPTIC CASA Systems, SCA class analyzer; Josep Tarradellas, Spain). Samples with ≥ 70% motility, ≥ 80% viability, and a sperm concentration ≥ 100 × 106 cells/mL were pooled and used for further processing.
Semen cryopreservation and thawing
Sperm cryopreservation and post-thaw quality assessment were performed following previously reported protocols [20] and are described in detail in this paper. Pooled sperm samples were adjusted to a concentration of 200 × 106 cells/mL with buffer 1. The sperm suspension was then divided and diluted with extender (buffer 2) supplemented with 100, 200, 400 µM RSV, or no RSV (control). Semen was extended in a multi-step dilution process to a final concentration of 100 × 106 cells/mL. Diluted semen was used to fill 0.5 mL semen straws (Ref. 13408/0010: Minitüb GmbH, Germany), which were then equilibrated at 4°C for 45 to 60 minutes. Freezing of the semen straws was performed by horizontally placing the straws 2 cm above the surface of liquid nitrogen for 15 minutes. After storage in liquid nitrogen for a week, the frozen semen straws were thawed in a 37°C water bath for 30 seconds for evaluation.
ROS level assessment
Sperm ROS levels were assessed according to the protocol described by Guthrie and Welch [28]. 2,7‐dichlorodihydrofluorescein diacetate (H2DCFDA; Molecular Probes Inc., USA) was used to detect H2O2. The mean fluorescence intensity [29] of DCF was measured to evaluate the intracellular mean H2O2 per viable sperm population. All fluorescence signals of labeled sperm were analyzed using a BD Accuri C6 Plus flow cytometer (BD Biosciences, USA). The data are expressed as the percentage of viable sperm with H2O2 (high DCF fluorescence).
Apoptosis status assessment
The sperm apoptosis status was evaluated according to Mehdipour et al. [30]. Sperm were washed in Dulbecco’s Phosphate Buffered Saline and readjusted to 100 mL at a concentration of 1 × 106 sperm/mL in 1 × binding buffer (10 mM HEPES/NaOH, pH 7.5, containing 0.14 M NaCl and 2.5 mM CaCl2), and 5 µL annexin V fluorescein isothiocyanate (FITC) (50 µg/mL stock) and 5 µL propidium iodide (PI) solutions were added. The cells were incubated for 15 minutes at room temperature in the dark. Sperm were categorized into 4 different groups: viable non-apoptotic, early apoptotic, late apoptotic, and necrotic cells. The last group contained late apoptotic and necrotic cells, which were categorized as dead cells. The apoptosis index was calculated as the ratio of annexin V detected among live sperm.
Statistical analysis
The IBM SPSS Statistics ver. 24.0 (IBM Corp., USA) was used to analyze the data. Apoptosis status and ROS levels were analyzed using bivariate correlation coefficients. The measurements of different parameters were expressed as the mean ± standard error of the mean, and p < 0.05 indicating statistical significance.
Results
ROS level
ROS level is calculated on the bases of the proportion of ROS detected sperm from total number of live sperm. In the quadrant, the upper and lower parts indicate the presence or absence of PI staining, and depending on this result, the sperm is categorized as live or dead. In addition, the left and right sides of the quadrant indicate is on the bases of DCFDA staining, which indicates the higher level of ROS {lower right quadrants / (lower left quadrants + lower right quadrants)}. The results of showed that the ROS level gradually decreased in the group treated with RSV at increasing concentrations (p < 0.05) (Fig. 1). In particular, there was a significant difference between the control group and the RSV 400 µM treatment group (measured as 4.31 ± 0.51 and 1.43 ± 0.18 in control and 400 µM RSV, respectively).

Effect of resveratrol (RSV) on reactive oxygen species (ROS) level of post-thaw sperm. (A) Control, (B) RSV 100, (C) RSV 200, (D) RSV 400. (A-D) are the results of DCFDA/propidium iodide staining and ROS analysis by flow cytometry. The bar graph is the result of comparing the ROS index value using the flow cytometry result. UL, upper left; UR, upper right; LL, lower left; LR, lower right.
*significant differences in means between each group and control group (p < 0.05).
Phosphatidylserine translocation status (apoptosis)
The apoptosis index was calculated as the probability that annexin V were detected depending on the number of live sperm. In the quadrant, the upper and lower parts indicate the presence or absence of PI staining, and depending on this result, the sperm is categorized as live or dead. In addition, the left and right sides of the quadrant indicate the presence or absence of annexin V FITC staining, which indicates the expression of apoptosis {lower right quadrants / (lower left quadrants + lower right quadrants)}. The apoptosis index showed a marked difference between the RSV-treated groups and the control group (p < 0.05) (Fig. 2). RSV supplementation at 400 µM in the cryoprotectant extender resulted in decreased apoptosis index (measured as 5.65 ± 0.58 and 3.29 ± 0.21 in control and 400 µM RSV, respectively).
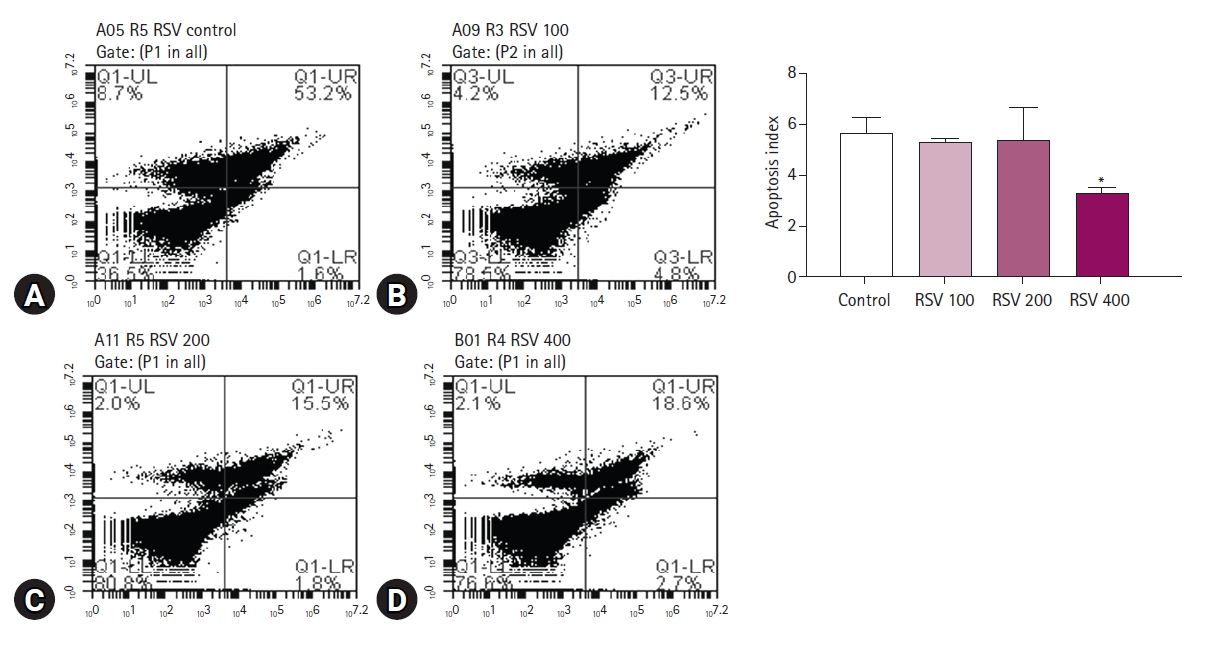
Detection of annexin V fluorescein isothiocyanate (FITC) in post-thaw porcine sperm. (A) Control, (B) resveratrol (RSV) 100, (C) RSV 200, (D) RSV 400. (A-D) are the results of annexin V FITC/propidium iodide staining and analysis by flow cytometry. UL, upper left; UR, upper right; LL, lower left; LR, lower right. *Significant differences in means between each group and control group (p < 0.05).
Discussion
RSV is an activating factor of SIRT1, which is responsible to reduces and improves ROS levels and there by reduces apoptosis in cells [31]. The flow cytometry method of apoptosis index and ROS determination of post-thaw sperm quality reveled relatively lower apoptosis index and ROS level at 400 µM RSV supplemented group is statistically significantly different than control (p < 0.05). The finding of current study, optimum concentration of RSV, as 400 µM in dog post-thaw sperm is higher than, 40 μM RSV reported to be efficient in roosters [32], which could be due to species-specific differences and usage of different media types. RSV has been shown to affect autophagy factor 1, which is an outer mitochondrial membrane protein, responsible for calcium metabolism, anti-apoptosis, and anti-autophagy [33,34]. Therefore, supplementation with RSV will up-regulate the development of mitochondrial organisms, allowing sperm to obtain the energy source required for activity after thawing [35].
The conventional sperm quality evaluation methods analyzes mainly cell membrane, mitochondria, DNA and acrosome and so far used in combination as a useful approach to determine the sperm quality [36]. Whereas advances in molecular cytometry enable to analyze sperm quality objectively and in relatively faster rate. These findings are similar to previous reports where apoptotic biomarkers were used to determine the male fertility potential [37]. Therefore, the excessive ROS levels in the control group could be responsible for physical and chemical stress on the sperm and affect fertility quality. This finding is in line with a previous report demonstrating that antioxidant supplementation using kinetin maintains the motility and survival rate of cryopreserved dog sperm [20].
The lower apoptosis index in sperm groups treated with RSV is an indication of better survival of the sperm in the treated groups as compared to that of the control group. In general, apoptosis is gene-activated and occurs as a normal consequence of cell aging or as a result of cellular stress [38]. The reduced apoptotic index in the treated groups is a mechanism to reduce the cryo-induced damage of the apoptotic process that occurs during freezing and thawing. This, in turn, is believed to contribute to maintaining the fertility quality of post-thaw sperm. On the other hand, high levels of ROS are a major cause of DNA damage that may result in genetic mutations and consequently different pathological conditions including aging, leading to apoptosis and cancer [39]. Damage to sperm DNA affects various fertility parameters [40].
RSV lowers the apoptotic index and ROS levels and should be considered as an effective antioxidant for post-thaw dog sperm at 400 µM. Furthermore, flow cytometry methods are effective for post-thaw sperm quality analysis, revealing similar results as those of conventional sperm motility and survival tests. We encourage further studies in this regard, particularly on the effects of higher concentrations of RSV and a wide-scale validation of the results of this study. Finally, we note that the study species and the types of cryopreservation media used are important factors to consider while determining the optimum concentration of RSV.
Notes
The authors declare no conflict of interest.
Acknowledgements
This work was supported by research fund of Chungnam National University.
