Splenic smooth muscle tumors in 7 dogs: case reports
Article information
Abstract
We describe 7 cases of splenic smooth muscle tumors in dogs. Histopathological and immunohistochemical analysis revealed that 6 cases were diagnosed as splenic leiomyosarcoma and 1 case as leiomyoma. All dogs underwent splenectomy without chemotherapy, and one of them was euthanized 2 months after surgery because of hepatic metastasis. Of the remaining 6 dogs, 5 died in the postoperative period and only one dog survived > 4 years. The median survival of the 6 dogs was 16.9 months. To the best of our knowledge, this is the first detailed study on splenic smooth muscle tumors in dogs in Korea.
Splenic masses are frequently observed in dogs. They may be diagnosed in dogs that present with non-traumatic hemoabdomen or diagnosed incidentally upon imaging or surgery [1]. Spleen tumors are generally classified into vascular tumors and mesenchymal tumors. A diverse array of primary mesenchymal tumor types occurs in the spleen and they mostly affect dogs. Among these, stromal tumors of the spleen are a heterogeneous group that share spindle cell morphology and are considered as primary splenic mesenchymal neoplasms. Malignant stromal tumors are more common than benign tumors and include fibrosarcoma and leiomyosarcoma most frequently and liposarcoma, myxosarcoma, rhabdomyosarcoma, chondrosarcoma, osteosarcoma, and mesenchymoma less frequently [2]. Reported benign stromal tumors are fibromas, lipomas, myelolipomas, and leiomyomas, which are all rare [3,4].
In the spleen, leiomyoma and leiomyosarcoma originate from smooth muscle, and they may arise from the blood vessel wall, trabeculae, or capsules in the spleen [5]. However, the occurrence of primary leiomyoma and leiomyosarcoma in the spleen is considered rare [6]; thus, reports on splenic neoplasia in dogs are extremely scarce. The objective of this study was to describe the clinical signs, histopathological diagnosis, and outcomes in dogs that underwent splenectomy for histologically confirmed splenic smooth muscle tumors.
The cases were obtained from the Department of Veterinary Pathology at the Jeju National University, South Korea, between January 2012 and December 2020. A total of 12 cases of splenic leiomyoma/leiomyosarcoma were diagnosed, and only 7 cases with clinical history and follow-up data were included in this study. The collected data were as follows: breed, age, sex, clinical signs at the time of diagnosis, treatment method, and outcome (distant metastasis and survival time).
Hematoxylin and eosin staining was performed using standard protocols on 4 µm sections from splenic tissues that had been fixed in 10% neutral buffered formalin, processed routinely, and embedded in paraffin wax. Replicate sections were also stained with special stains, such as Masson’s trichrome stain, to determine the origin of these tumors. In addition, to clarify the origin of neoplastic cells, immunohistochemistry was performed on splenic tissues using mouse monoclonal anti-Desmin (1:100 dilution; Dako, Denmark) and mouse monoclonal anti-smooth muscle actin (SMA) (1:100 dilution; Dako).
The signalment, clinical history, treatment, and metastatic informations are summarized in Table 1. All 7 dogs were aged 7 to 14 years (mean, 9.7 years) and included 4 castrated males, 1 neutered female, and 2 intact females of 4 different breeds. The represented breeds were Yorkshire Terrier (case 1), Cocker Spaniel (cases 2 and 4), Shih Tzu (cases 3, 6, and 7), and Poodle (case 5). Clinical signs at the time of presentation included anorexia (n = 4, 57.1%), lethargy (n = 2, 28.6%), abdominal pain (n = 1, 14.3%), ataxia (n = 1, 14.3%), and diarrhea (n = 1, 14.3%). The presence of a splenic mass was incidentally found in 5 dogs. Of these, 3 dogs were presented because of anorexia, and an abdominal mass was incidentally palpated. Abdominal radiography was performed in all dogs, and a mass was detected in all 7 dogs. On radiography and ultrasonographic evaluation, pulmonary nodules in 1 dog (case 2) and liver nodules in 2 dogs (cases 2 and 6) were noted, which were suspected to be metastatic neoplastic foci.
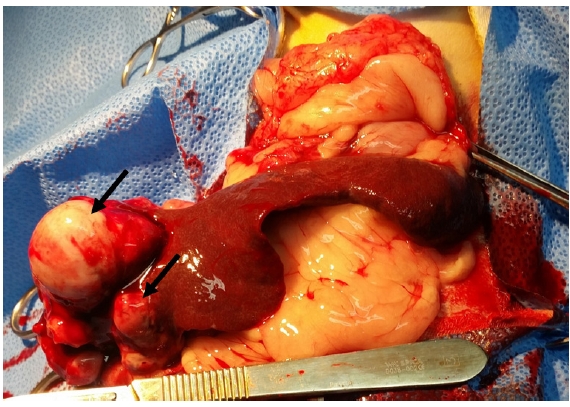
Splenectomy was performed in all 7 dogs, and the splenic samples were examined grossly. Grossly, all cases had one or several large protruding splenic masses (Fig. 1). Neoplastic foci were moderate to rubbery in terms of firmness and had smooth surfaces. The masses varied in diameter from 2 cm to 4 cm. These well-circumscribed masses were yellowish-white in color and had focal to multifocal dark red foci on the cut surface.
The histological features of these cases were similar, except for 1 case (case 1). In case 1, the neoplastic foci were composed of densely or loosely packed spindle cells and were partially surrounded by fibrous connective tissue. The neoplastic cells were well-differentiated spindle-shaped cells resembling smooth muscle cells (Fig. 2A). These ovoid cells had moderate to large amounts of eosinophilic cytoplasm with elongated and fusiform nuclei. Other cases (cases 2-7) showed more pleomorphic histological features than case 1. The neoplastic spindle cells arranged in multidirectional bundles (Fig. 2B). These abnormal cells with high cellular density, intense anisocytosis, and anisokaryosis had eosinophilic cytoplasm with central or eccentric nuclei. These cases had diverse pleomorphisms and mitotic figures in the mass (Fig. 2C). In 4 of 6 malignant cases, tumor cells originated from the vascular tunica media (Fig. 2D). Multifocal necrosis and inflammation were also observed in the mass.

Histological findings. (A) Case 1. Spindle-shaped neoplastic cells with non-atypical nuclei and eosinophilic cytoplasm. Hematoxylin and eosin staining (H&E), scale bar = 20 µm. (B) Case 2. Neoplastic foci were composed of densely packed spindle cells and formed broad, interlacing fascicles. H&E, scale bar = 100 µm. (C) Case 4. Numerous mitotic figures (arrows) were observed in neoplastic area. H&E, scale bar = 20 µm. (D) Case 6. Tumor cells originating from the vascular tunica media (arrow), which is composed of smooth muscle cells. H&E, scale bar = 100 µm. (E) Case 5. Neoplastic cells were stained with red in Masson’s Trichrome stain. Scale bar = 100 µm. Case 3. Neoplastic spindle cells were demonstrated strong positive reactions to desmin (F) and smooth muscle actin (G). IHC, scale bar = 50 µm.
In Masson’s trichrome staining, neoplastic cells in all cases were stained red (Fig. 2E). Immunohistochemically, the neoplastic spindle cells demonstrated strong positive reactions for desmin (Fig. 2F), a muscle cell marker; and SMA (Fig. 2G), a smooth muscle cell marker.
None of the dogs received adjunctive therapy. One of the two dogs with metastatic disease was euthanized 2 months after surgery, and the other died 3 months after surgery, which was suspected to be a disease-related death. Four dogs died because of unrelated diseases several weeks, 1 year, 3 years, and 4 years after surgery, respectively. Only one dog was alive and healthy for 2 years. The overall median survival time (MST) was 16.9 months (range, 0.5 to 48 months).
Herein, we describe 7 cases of splenic leiomyomas and leiomyosarcomas. Gross, histopathological, and immunohistochemical findings supported the diagnosis of smooth muscle tumors. To the best of our knowledge, this is the first detailed study on splenic smooth muscle tumors in dogs in South Korea.
In a previous study, there was no breed or sex predilection of splenic leiomyosarcoma [2]. In this study, the median age of dogs was 9.7 years. This was similar to that in a previously reported study for splenic sarcomas [2,7].
Splenic stromal sarcomas include various types of tumors, such as fibrosarcoma, leiomyosarcoma, liposarcoma, myxosarcoma, rhabdomyosarcoma, chondrosarcoma, osteosarcoma, and mesenchymoma. Tumors composed of spindle-shaped cells are difficult to differentiate by histopathologic examination alone. Therefore, immunohistochemistry is required to classify the type of tumor. In this study, we used a special staining method and immunohistochemistry to determine the origin of tumor cells. All cases were tumors of smooth muscle cell origin; therefore, these cases could be differentiated from other sarcomas.
The most common clinical signs of splenic leiomyomas or leiomyosarcomas in dogs were anorexia, lethargy, abdominal pain, ataxia, and diarrhea. Gastrointestinal tract signs may indicate a large splenic mass that has displaced the abdominal viscera [8]. Moreover, abdominal pain or distention may occur secondary to tumor mass and/or peritoneal effusion. Although these clinical signs could help confirm the diagnosis, they were not diagnostic for splenic leiomyosarcoma. Therefore, radiographic examination and histopathology should be performed to diagnose abdominal tumors.
Total splenectomy is the treatment of choice for splenic leiomyosarcoma [9]. In a previous study, the prognosis of dogs treated surgically for leiomyosarcoma of the spleen, stomach, and small intestine was good to excellent. However, dogs with leiomyosarcoma of the liver had a poor prognosis [7]. In this study, splenectomy without additional chemotherapy was performed in all dogs at the time of confirmation for splenic tumors using radiographic examination. After surgery, only one dog was still alive, and 6 dogs were euthanized or died due to disease. According to previous studies, the MST for dogs with splenic leiomyosarcoma is 8 months [10] or 4 months [11]. In this study, the MST was longer than that in other reported cases, although the dogs underwent surgical resection of splenic mass without post-surgical chemotherapy.
The presence of a concurrent hepatic mass with a splenic mass has been reported to be associated with metastatic disease [12]. In a previous study, the most common metastatic site for splenic sarcoma in dogs was the liver [2]. Splenic neoplasms commonly spread via the splenic and portal veins to the liver and subsequently through the systemic circulation [8]. Two of the seven dogs showed abnormal hepatic masses in the parenchyma. These dogs had a poorer prognosis than those without metastasis.
Postoperative monitoring is important because of the frequency of postoperative mortality in dogs with splenic sarcomas [2]. Patients undergoing splenectomy should be observed for signs of shock, anemia, ventricular arrhythmias, and disseminated intravascular coagulopathy. Moreover, most dogs with a solitary mass died or were euthanized within several months after splenectomy because of metastatic disease. Therefore, abdominal ultrasonography and computed tomography may be used to monitor the spread of these neoplasms before and after splenectomy [2].
Notes
The authors declare no conflict of interest.
Acknowledgements
This research was supported by the 2021 scientific promotion program funded by Jeju National University.