Feline progressive histiocytosis in four cats: case reports
Article information
Abstract
We describe four cases of feline progressive histiocytosis (FPH) including three females (one intact, two spayed) and one castrated male cat, with a mean age of 5.95 years at diagnosis. Masses were found under the skin of head, lip, neck, and vulva. Histologically, proliferative round cells had ovoid nuclei, foamy eosinophilic cytoplasm, distinct cytoplasmic processes, and numerous mitotic figures. Immunohistochemically, all cases were positive for Iba1 and MHC II (Dako). One case showed cytoplasmic positive staining for E-cadherin. To the best of our knowledge, this is the first documented report of FPH in Korea.
Histiocytic disorders are uncommon in cats compared with dogs [1–3]. In cats, this group of diseases is classified into pulmonary Langerhans cell (LC) histiocytosis, histiocytic sarcoma (HS), and feline progressive histiocytosis (FPH) with indolent behavior. The latter was initially considered a low-grade HS [1]. Among these histiocytic disorders, FPH is the most common and invariably begins with cutaneous nodules and plaques. In FPH and HS, the origin of neoplastic cells is considered to be interstitial dendritic cells (iDCs), while LCs are usually associated with pulmonary LC histiocytosis [1,2,4,5]. However, in recently reported cases of FPH, the neoplastic cells were positive for E-cadherin, an LC marker [6,7]. Therefore, the origin of neoplastic cells in FPH remains controversial. FPH is common in middle-aged to older cats with about 7 to 17 years old [1]. The lesions are mostly located on the head, lower extremities, or trunk [1–3]. A predisposition to a particular sex and breed has not yet been reported. Several cases of FPH have been published worldwide; however, there are no official publications or available data for this particular tumor in Korea. This study describes the clinical, histopathologic, and immunohistochemical (IHC) characteristics of four cases of FPH.
A total of 449 feline cutaneous tumors were diagnosed between January 2014 and December 2021 at the Pathology Department of Veterinary Medicine, Jeju National University. Of these, four cases (0.89%) were diagnosed as FPH, which are reported here. Clinical data was obtained for these cases. For histopathologic analysis, the submitted tissues were fixed in 10% neutral buffered formalin, trimmed, embedded in paraffin wax, sectioned at 3 µm, and stained with hematoxylin and eosin. Special staining such as periodic acid-Schiff (PAS), Ziehl–Neelsen, and toluidine blue staining was also performed for the differential diagnosis of possible cutaneous granulomatous inflammation and histiocytic mast cell tumors. IHC analyses were performed to identify the origin of tumor cells using various antibodies: MHC II (1:40, monoclonal mouse, M0746; Dako, Denmark), Iba1 (1:1,000, polyclonal rabbit, 019-19741; Wako, Japan), E-cadherin (1:50, monoclonal mouse, M3612; Dako), CD3 (1:100, polyclonal rabbit, A0452; Dako), and Pax-5 (1:100, monoclonal mouse, 610863; BD, USA).
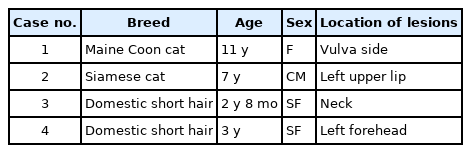
The detailed information of four cases including breed, age, sex, and anatomic location of the mass is summarized in Table 1. All cases had a solitary, round, protruding mass under the skin (Fig. 1A). The lesions were firm and alopecic in all cases. The masses were whitish in color with a smooth-cut surface, ranging in diameter from 7 to 17 mm. The four cases also shared similar histopathologic characteristics: The masses all contained neoplastic foci and numerous abnormal round cells, which accumulated in the area of the superficial to the deep dermis (Fig. 1B). These neoplastic cells had round-to-ovoid hyperchromatic nuclei, foamy eosinophilic cytoplasm, and distinct cytoplasmic processes (Fig. 1C). The mitotic figures ranged from 1 to 4 per high-power field (Fig. 1D). Anisocytosis and anisokaryosis were mild to moderate. Many adnexal structures adjacent of the mass were severely atrophied, and many parts of the collagenous tissue in the dermis showed moderate degeneration. A small number of lymphocytes and neutrophils multifocally infiltrated the lower part of the mass. PAS and Ziehl–Neelsen staining did not reveal the presence of microorganisms, and no obvious reaction was observed in the neoplastic cells after toluidine blue staining.
Gross and histopathologic findings (A) Case 2. A solitary round protruding mass in the left upper lip. (B) Case 4. Neoplastic round cells infiltrated in the dermis without infiltration of the epidermis. H&E, scale bar: 100 µm. (C) Case 4. Neoplastic cells show round to oval shape and have abundant eosinophilic cytoplasm. H&E, scale bar: 50 µm. (D) Neoplastic round cells have ovoid nuclei, eosinophilic foamy cytoplasm, and mitotic figures (arrows). H&E, scale bar: 20 µm.
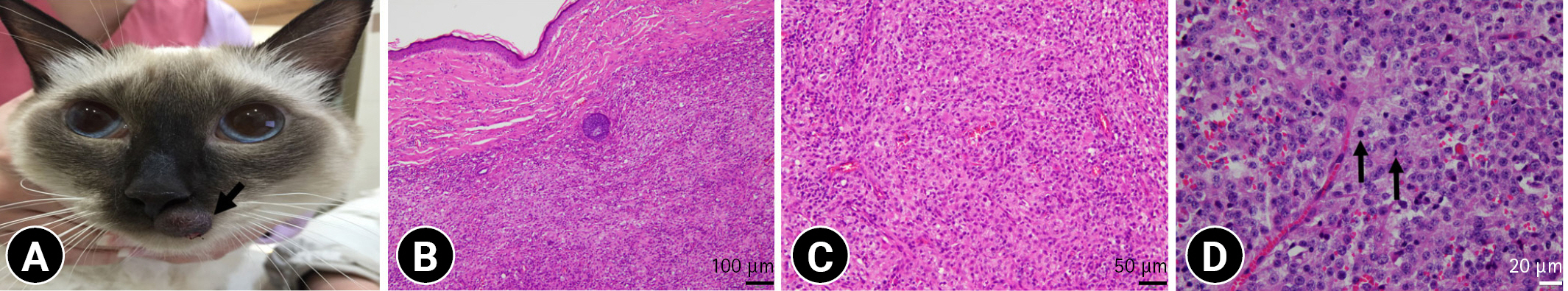
IHC analysis showed that neoplastic round cells in all cases were strongly positive for MHC II (Fig. 2A) and Iba1 (Fig. 2B). In tissue samples of case 1, the neoplastic cells showed diffuse cytoplasmic positivity for E-cadherin (Fig. 2C). The expression of CD3 and Pax-5 was not detected in neoplastic cells of all cases, except multifocally infiltrated lymphocytes.
Histological findings. Neoplastic round cells in all cases show positive reactions for MHC II (A) and Iba-1 (B). (C) In case 1, neoplastic cells show strong positive reactions to E-cadherin. Immunohistochemical, scale bars: 50 µm.
Genarally, there is no predisposition regarding age or breed in FPH, and the small number of cases included in this study poses a limitation for the inference of any kind of predisposition. However, a previous study reported that females had a greater prevalence of FPH compared with males [3]. Moreover, the masses were most common on the head and neck [1–3]. The anatomic location and sex of the herein studied individuals are similar to those in the previous studies.
The major differential diagnoses of these cases are mycobacterial and fungal diseases, or round cell tumors, including histiocytic mast cell tumor, plasmacytoma, and lymphoma. For the differential diagnosis of possible granulomatous inflammation, PAS and Ziehl–Neelsen staining were used. No case reacted to the staining; hence, infections by microbial pathogens could be successfully ruled out. Histological analysis showed that the cutaneous masses in all cats were composed of proliferated round cells with abundant cytoplasm. Mild-to-moderate cytological atypia was observed, although without epithelial involvement. Based on histological findings, these cases were tentatively diagnosed as feline round cell tumors.
To identify the origin of tumor cells, IHC analyses were performed. Using histiocytic markers in IHC analyses is a common method to identify the origin of tumor cells [3,4,6,7]. The skin includes dermal DCs and epidermal LCs [2]. Dermal DCs are interstitial cells that can be encountered in perivascular locations in many organs [3]. On the other hand, LCs populate the epidermis and epithelium of the mucous membrane. Both cell types express CD1, CD11c, MHC II, and Iba1; however, E-cadherin is not expressed in dermal DCs [2]. Iba1, a protein involved in the rearrangement of the actin cytoskeleton, has previously been used to detect the subpopulations of cells of monocyte/macrophage lineages in mice [8]. Iba1 is expressed not only in histiocytes that cause FPH and HS but also in LCs and dermal histiocytes of healthy individuals [9]. In contrast, plasmacytomas, cutaneous lymphomas, and mast cell tumors do not express Iba1 [9]. The MHC II antibody labels B cells, activated T cells, macrophages, and antigen-presenting cells, such as LCs [10]. Therefore, we ruled out the following tumors: plasmacytomas, cutaneous lymphomas, and mast cell tumors. E-cadherin is a glycoprotein with an extracellular amino terminus that binds selectively to an identical E-cadherin amino terminus on an adjacent cell. It is expressed in epithelia and organs derived from epithelia throughout the body [1,11]. E-cadherin is present on the surface of basal and suprabasal keratinocytes in the skin. In mice and humans, LCs have also been shown to express high levels of E-cadherin [11]. In this study, histiocytic origin of the cells was confirmed in all four cases by diffuse immunoreactivity for MHC II and Iba1. Among the four cases, the neoplastic cells of one case were also immunoreactivity for E-cadherin, an LC marker. In previous studies of FPH, 4/5 and 24/26 cases were positive for E-cadherin [6,7]. Further studies are needed to definitively confirm the LC versus iDC origin of FPH.
The initial presentation of FPH may be a solitary skin nodule, although multiple papules, nodules, or plaques may develop [1]. In FPH, a proportion of cats develop invasive expansile masses in the lymph nodes and internal organs, including the lungs, kidneys, spleen, and liver, similar to HS [1,12]. In this study, all cats had a solitary skin nodule and did not show any evidence of tumor metastasis at the time of diagnosis. Follow-up data were collected from cases 2 and 3. One cat died because of an unrelated disease for FPH 4 years after surgery. The other cat showed abnormal nodules in the same region where the FPH-related tumor had been found; however, the nodule was diagnosed as another type of tumor.
The early identification of tumor types can facilitate prognosis and treatment. It is important to confirm the diagnosis using histopathological and IHC tests because feline histiocytic diseases behave differently from each other [13]. To the best of our knowledge, this is the first retrospective study of FPH in South Korea. Although the occurrence of FPH is low, clinicians and pathologists should consider this rare entity for differential diagnosis of feline cutaneous tumors.
Notes
The authors declare no conflict of interest.
Acknowledgements
This research was supported by the 2022 scientific promotion program funded by Jeju National University.