Evaluation of commercial immunochromatography test kits for diagnosing canine parvovirus
Article information
Abstract
Rapid immunochromatography test (RICT) kits are commonly used for the diagnosis of canine parvovirus (CPV) because of their rapid turnaround time, simplicity, and ease of use. However, the potential for cross-reactivity and low sensitivity can yield false-positive or false-negative results. There are 4 genotypes of CPV. Therefore, evaluating the performance and reliability of RICT kits for CPV detection is essential to ensure accurate diagnosis for appropriate treatment. In this study, we evaluated the performance of commercial RICT kits in the diagnosis of all CPV genotypes. The cross-reactivity of 6 commercial RICT kits was evaluated using 8 dog-related viruses and 4 bacterial strains. The limit of detection (LOD) was measured for the 4 genotypes of CPV and feline panleukopenia virus. The tested kits showed no cross-reactivity with the 8 dog-related viruses or 4 bacteria. Most RICT kits showed strong positive results for CPV-2 variants (CPV-2a, CPV-2b, and CPV-2c). However, the 2 kits produced negative results for CPV-2 or CPV-2b at a titer of 105 FAID50/mL, which may result in inaccurate diagnoses. Therefore, some kits need to improve their LOD by increasing their binding efficiency to detect all CPV genotypes.
Introduction
Canine parvovirus (CPV) is a small, non-enveloped, single-stranded, linear DNA virus with a diameter of 18 to 26 nm. It causes acute hemorrhagic gastroenteritis, vomiting, diarrhea, leukopenia, and fetal myocarditis in puppies [1]. CPV belongs to the genus Protoparvovirus within the family Parvoviridae. The CPV genome is approximately 5 kb in length and encodes 2 open reading frames, VP1 and VP2 [2]. The VP2 protein contains antigenic epitopes that determine its antigenicity and host range [3]. Over the years, CPV has evolved into 4 different genotypes, namely CPV-2, CPV-2a, CPV-2b, and CPV-2c. These genotypes differ from each other in their amino acid sequences, particularly in the VP2 [4]. CPV-2a was the first identified variant of CPV-2 and was responsible for the initial outbreaks of CPV in the 1970s [5]. CPV-2b emerged in the 1980s and quickly became the predominant strain, whereas CPV-2c was first detected in Italy in the late 1990s and has since spread to other countries [5,6]. In South Korea, CPV-2a and CPV-2b were detected between 2003 and 2006, and CPV-2c was also detected in 2020 [7,8].
Rapid and accurate diagnosis of parvoviral infection is crucial for timely and appropriate treatment, as well as preventing the spread of the disease to other animals [9]. Several laboratory methods are available for the detection of CPV in infected dogs, including the rapid immunochromatography test (RICT), hemagglutination assay (HA), immunohistochemistry, virus isolation (VI), and polymerase chain reaction (PCR)-based methods [9]. RICT is a commonly used method in veterinary clinics. This test provides results within 10 min and does not require special equipment or expertise [10]. HA and VI have been used as laboratory methods to detect CPV; however, they can only be performed in laboratories equipped with reagents and facilities [11,12]. PCR-based methods, particularly real-time PCR, are more sensitive and specific than other techniques but require expensive equipment and skilled operators [13,14]. RICT kits have become an essential tool for diagnosing parvovirus infections in dogs, and several kits are available in the market. However, these may give false-negative results because of their low limit of detection (LOD) [10]. These kits have been designed to detect CPV antigens in the fecal samples of infected dogs using immunochromatographic techniques [15]. These kits use monoclonal antibodies that are specific to CPV and can detect CPV with no cross-reactivity and high LOD [15].
Owing to the emergence of various CPV genotypes in recent years that differ from those in the past, we conducted a re-evaluation of the RICT kits. By examining the cross-reactivity and LOD performance of multiple commercial RICT kits, we aimed to ensure their reliability and accuracy in detecting diverse CPV genotypes.
Materials and Methods
Viruses and bacteria
RICT kits for examining cross-reactivity and LOD were purchased, and the pathogens to be applied to the RICT kit were obtained from the Animal and Plant Quarantine Agency (Korea) and American Type Culture Collection (ATCC; USA). Cross-reactivity was tested using the following pathogens: canine adenovirus-1 (CAV-1) ATCC VR293, canine adenovirus-2 (CAV-2) KVCC VR2000005, canine respiratory coronavirus (CRCoV) KVCC VR2300007, canine distemper virus (CDV) KVCC VR2200060, canine parainfluenza virus type 5 (CPIV-5) ATCC VR399, rabies virus (RABV) KVCC VR190060, canine influenza virus (CIV) KVCC VR1300034, rotavirus (RoV) KVCC VR1600036, all of which have a titer of at least 105 TCID50/mL, in addition to Bordetella bronchiseptica KVCC BA1800109, Staphylococcus aureus KVCC BA2100324, Staphylococcus epidermidis KVCC BA2100206, and Escherichia coli KVCC BA2100174, all of which had a colony-forming unit of at least 106. The LOD was tested using the following pathogens: CPV-2 ATCC VR953, CPV-2a KVCC VR2200065, CPV-2b KVCC VR1500038, CPV-2c KVCC VR2000013, and feline panleukopenia virus (FPV) KVCC VR2100012.
Rapid immunochromatographic test kits
Six RICT kits were used, including the Canine Parvovirus Test Kit (VetAll, Korea), CPV Ag Rapid Kit (Median Diagnostics, Korea), CCV/CPV Ag (GenBody, Korea), Anigen Rapid CPV Ag Test Kit (BioNote, Korea), CPV Ag Test (RapiGEN, Korea), and Asan Easy Test Parvo (ASAN Pharm, Korea).
The LOD of the 6 RICT kits were determined by testing with the various concentrations of the 4 CPV genotypes and FPV. Six RICT kits were used following the manufacturer’s instructions. Briefly, each virus or bacterium was diluted 1:1 with the diluent provided with the kit. Then, 100 µl of the mixture was taken and dropped onto the test device. The intensity of the positive bands was measured with the naked eye after 10 to 15 minutes. Positive bands were scored as judged by eye: 3, strong; 2, medium; 1, weak; 0.5, very weak; 0, negative [16].
Analysis of the VP2 gene
For phylogenetic tree analysis, the VP2 nucleotide sequences of CPV and FPV used in this study were analyzed along with the nucleotide sequences of the CPV and FPV reference strains. Nucleotide sequences of VP2 of CPV-2 (NCBI-FJ197847), CPV-2a (NCBI-OP972595), CPV-2b (NCBI-KP893077), CPV-2c (NCBI-MH764263), FPV (KVCC- VR2100012) were used as representatives of the 4 genotypes of CPV and FPV. CPV-2 (n = 6), CPV-2a (n = 9), CPV-2b (n = 10), CPV-2c (n = 9), and FPV (n = 4) were obtained from the National Center for Biotechnology Information (NCBI, USA) and used as reference strains. A phylogenetic tree was constructed using the Bayesian evolutionary analysis sampling trees (BEAST) v.1.10.4 software [17]. The phylogenetic tree was constructed using Figtree v.1.4.4. The amino acid sequences of VP2 were aligned using BioEdit v.7.2 software.
Results
Cross-reactivity and LOD
Six RICT kits were tested for cross-reactivity with 8 viruses (CAV-1, CAV-2, CRCoV, CDV, CPIV-5, RABV, CIV, and RoV) and 4 bacteria (B. bronchiseptica, S.aureus, S.epidermidis, and E.coli), and no cross-reactivity was observed. The LODs for CPV-2, CPV-2a, CPV-2b, CPV-2c, and FPV were within the range of viral titers of 106 to 104 FAID50/mL (Table 1). The results showed that all kits were positive for CPV-2a and CPV-2c. In contrast, kits A and C displayed a negative reaction to CPV-2 at a viral titer of 106, and FPV also showed a negative reaction. In addition, kit F showed a negative reaction for CPV-2b (Table 2). The total score for the detection strength of the kits revealed significant differences among the 4 CPV genotypes. As shown in Table 2, the CPV genotype showing the highest detection strength was CPV-2a (17 scores) and the lowest was CPV-2 (11 scores) at a viral titer of 106 FAID50/mL.
Genetic analysis of the VP2 gene
A similarity analysis was conducted for the nucleotide sequences of the VP2 gene of the CPV and FPV used in this study, and the results showed that CPV had very high similarity ranging from 97.9% to 99.4% (Fig. 1A). Following similarity analysis, CPV-2 exhibited a nucleotide sequence similarity of 98.6% with FPV, whereas CPV-2a and CPV-2b showed the highest nucleotide sequence similarity of 99.4%. Phylogenetic analysis was performed using a dataset that included 43 VP2 nucleotide sequences of CPV and FPV. This analysis showed that CPV was divided into 6 clades (Fig. 1B).
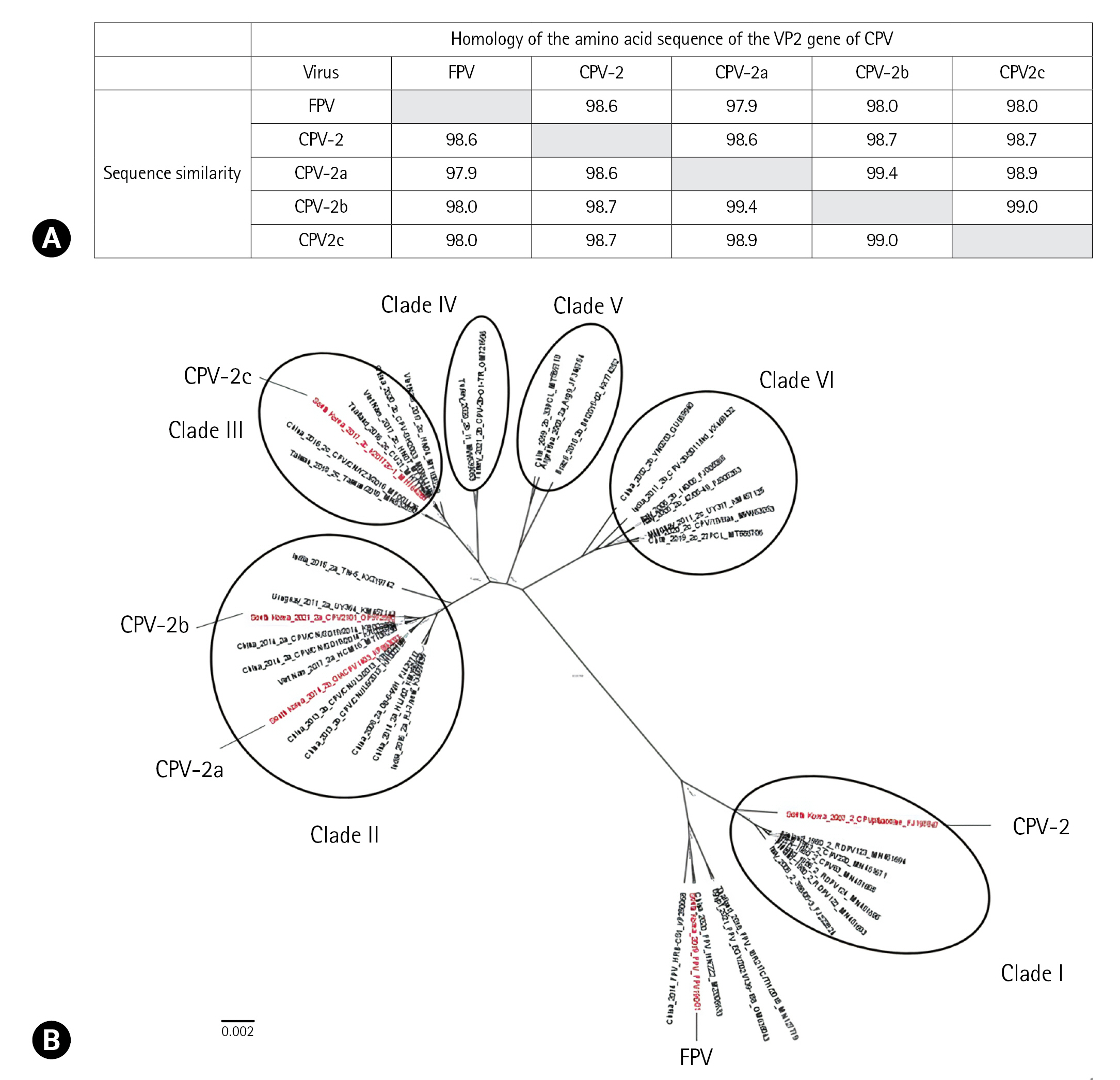
Alignment of amino acid sequences of the VP2 protein of the canine parvovirus strains and feline panleukopenia virus (A) and phylogenetic tree based on the VP2 gene of 38 canine parvovirus (CPV) strains and 5 feline panleukopenia (FPV) strains (B). The black circles represent CPV clades, and CPV was divided into 6 clades. Strains of the representative 4 genotypes of CPV and FPV used in this study are indicated in red.
Discussion
CPV is a highly contagious viral disease that can be fatal to puppies. Rapid and accurate diagnosis of CPV infections can lead to appropriate disease management [1]. RICT kits are commonly used in veterinary clinics and laboratories to diagnose CPV diagnosis [10]. Compared to other diagnostic methods, such as PCR, VI, and HA, the RICT kit offers several advantages, including rapid turnaround time, simplicity, and ease of use [10]. However, cross-reactivity with dog-related viruses and bacteria can lead to false-positive or false-negative results. This study evaluated the cross-reactivity of 6 commercial RICT kits for CPV with 8 dog-related viruses and 4 bacterial strains. The results showed that these kits had a specialized design for CPV detection and did not exhibit cross-reactivity with the 8 dog viral pathogens and 4 bacteria, making them reliable for accurate CPV diagnosis. However, the possibility of cross-reactivity with other pathogens cannot be completely excluded, and further investigations using a diverse range of potential pathogens, such as canine herpesviruses, are required to comprehensively assess the performance of the kits and identify any potential limitations.
We also evaluated the LOD of the 6 RICT kits for CPV-2, CPV-2a, CPV-2b, CPV-2c, and FPV. The results showed that the kits had appropriate LOD for CPV-2a and CPV-2c at a titer of 105 FAID50/mL. However, discrepancies were observed in the competence of the RICT kits in detecting CPV-2 and CPV-2b. For CPV-2, kits A and C produced negative results, whereas, for CPV-2b, all kits except kit F produced positive results. Inconsistencies in the ability of RICT kits to detect CPV-2 or its variants can lead to inaccurate diagnosis and delays in appropriate treatment, thus negatively affecting animal health outcomes. To overcome this inconsistency in CPV-2, a monoclonal antibody covering the major epitope of the CPV VP2 protein was selected and used with the RICT kit. When reevaluating the RICT kit, evaluation using all CPV genotypes is required.
In contrast, evaluation of the LOD of the 6 RICT kits revealed that 4 of them showed positive results for FPV at a titer of 105 FAID50/mL. This is because CPV and FPV are members of the Parvoviridae family and share a high degree of genetic similarity, with CPV-2 believed to have originated from a mutation in FPV [18]. Furthermore, LOD analysis indicated that CPV-2 and FPV exhibited similar LOD patterns. This result can be attributed to the close genetic relationship between VP2 CPV-2 and FPV, as inferred from the phylogenetic tree analysis of VP2. CPV has been classified into 4 genotypes based on amino acid sequence analysis [2,4]. However, a Bayesian evolutionary analysis of CPV revealed that it can be divided into 6 groups, I, II, III, IV, V, and VI. Other studies have reported different classifications of CPV for the 4 genotypes [19,20]. In our study, we only examined the results for 3 clades of CPV: I, II, and III. However, further studies are required for clades IV, V, and VI, as they have not yet been tested. Therefore, it is necessary to further assess the remaining 3 clades to ensure a comprehensive diagnosis of CPV.
In conclusion, we evaluated the use of commercial RICT kits manufactured in South Korea for CPV testing. Six kits did not show cross-reactivity with dog-related viruses and bacteria and demonstrated moderate LOD for detecting CPV-2a, CPV-2b, and CPV-2c. However, discrepancies in the LOD of the RICT kit were observed based on the CPV genotype. Consequently, some commercial RICT kits may have difficulty detecting all CPV genotypes that have emerged. These findings indicate that improvements in the discriminatory competence of these kits are necessary to ensure the accurate detection of the various CPV genotypes circulating currently.
Notes
The authors declare no conflict of interest.
Acknowledgements
This work was supported financially by a grant (N-AD20-2010-19-01) from the Animal, and Plant Quarantine Agency, Ministry of Agriculture, Food and Rural Affairs, Republic of Korea.


