Evaluation of hemagglutination inhibition test for canine respiratory coronavirus antibodies and seroprevalence in Korean dogs
Article information
Abstract
Canine respiratory coronavirus (CRCoV) is a significant pathogen that causes respiratory diseases in dogs, collectively known as a canine infectious respiratory disease. The virus is highly contagious and exhibits high seroprevalence worldwide. Currently, bovine coronavirus (BCoV) enzyme-linked immunosorbent assay (ELISA) kits are used to detect CRCoV antibodies. However, BCoV-ELISA kits cannot differentiate between infections caused by BCoV and those caused by CRCoV. In this study, we evaluated the hemagglutination inhibition (HI) test for CRCoV by comparing it with the virus neutralization (VN) test. Subsequently, we evaluated the seroprevalence of CRCoV in 383 dog serum samples collected from South Korea utilizing the HI test. The HI test for CRCoV showed a strong correlation with the VN test (R = 0.83, p < 0.001). The analysis of seroprevalence revealed that 52.2% (95% confidence interval [CI], 47.2–57.1%) of the Korean dog serum samples were positive. The seroprevalence exhibited varied with age, with a positivity rate of 43.9% in dogs under 1 year of age and 66.7% in dogs aged 3 to 5 years (odds ratio, 2.54; 95% CI, 1.43–4.59). In conclusion, the HI test to monitor CRCoV antibody proved to be closely related to the VN test. Furthermore, over half of the dogs in Korea tested positive for CRCoV antibodies. These findings contribute to a better understanding of the sero-epidemiology of CRCoV.
Introduction
Canine respiratory coronavirus (CRCoV) was first discovered in 2003 in the United Kingdom, specifically in dogs with canine infectious respiratory disease (CIRD), commonly known as kennel cough [1]. This highly infectious disease primarily affects dogs in densely populated environments such as shelters and veterinary clinics. It typically manifests as a mild condition characterized by a dry, hacking cough. However, in some cases, it can progress to severe bronchopneumonia, potentially leading to fatal outcomes [2]. The pathogenesis of CRCoV is associated with single or multiple infections involving numerous viral and bacterial pathogens such as canine parainfluenza virus, canine adenovirus 2, canine herpes virus, and Bordetella bronchiseptica [3–6]. CRCoV is believed to play a role in the early stages of CIRD by impeding clearance in the upper airways, rendering dogs more susceptible to secondary infections [7]. Because CRCoV is widespread across North America, Europe, and Asia, the seroprevalence has been determined in various countries, including the United States, Canada, South Korea, New Zealand, and Japan [8–11]. Among these countries, Canada and the United States reported high seroprevalence rates, with 59.1% and 54.7% of tested sera yielding positive results, respectively [8]. In contrast, South Korea recorded a CRCoV seroprevalence rate of 12.8% in 2010 [9]. Several serological tests, including virus neutralization (VN), indirect fluorescent antibody (IFA), and enzyme-linked immunosorbent assay (ELISA), have been developed to detect CRCoV infections [12]. Among these, ELISA utilizing the bovine coronavirus (BCoV) antigen has been widely utilized for CRCoV serological testing [8,10]. This approach is feasible because of the high amino acid sequence similarity (up to 96.0 %) between the spike proteins of CRCoV and BCoV [1]. In contrast, canine enteric coronavirus (CECoV) exhibits only 21% amino acid sequence identity with the spike protein, indicating low cross-reactivity. Additionally, the CECoV vaccine does not confer protection against CRCoV infection [12]. ELISA utilizing the CRCoV antigen has demonstrated slightly higher sensitivity and specificity than BCoV-ELISA, and both ELISA tests have shown an overall high agreement in results [8]. Despite the marked similarity in spike proteins, the hemagglutination inhibition (HI) test based on the BCoV antigen has shown poor sensitivity and specificity compared to BCoV-ELISA [13]. VN and IFA are accurate and useful for antibody detection; however, they are time-consuming and costly. The HI test is preferred because it does not require cell culture and is more economical than the ELISA. Currently, there are no reports of HI tests for CRCoV infection. Therefore, HI tests are required for the serological monitoring of CRCoV.
In this study, we aimed to evaluate the suitability of the CRCoV antigen-based HI test for the measurement of CRCoV antibodies and compare it with the VN test. This test was used to investigate the seroprevalence of CRCoV in the sera of 383 dogs in South Korea. Furthermore, we analyzed any possible associations between CRCoV seroprevalence and factors such as age, sex, and sample collection year.
Materials and Methods
Sample collection and virus
In total, 383 blood samples were collected from dogs residing in Gyeonggi and Seoul provinces of South Korea between 2019 and 2022. Dogs were randomly selected to ensure a diverse representation of the dog population. The dog’s health status at the time of blood collection showed no clinical problems with its respiratory system. None of the dogs were inoculated with the CRCoV vaccine. This vaccine has not yet been commercialized in South Korea. Approximately 3 to 5 mL of blood was collected from each dog and transferred to sterile plain vacutainer tubes. Blood samples were allowed to clot at room temperature for 30 min, followed by centrifugation at 3,000 rpm for 10 minutes to separate the serum. The collected serum was then aliquoted into labeled cryovials and stored at −20°C until further analysis. The BCoV QIABC0516 (KVCC-VR1500040) and CRCoV SNU_V22-9 (KVCC–VR2300007) were obtained from the Animal and Plant Quarantine Agency (Gimchen, Korea).
Virus identification
For the CRCoV identification, the virus was cultivated using HRT-18 cells (ATCC CCL-244, USA) in Dulbecco’s modified eagle medium (DMEM) supplemented with penicillin (100 U/mL), streptomycin (100 μg/mL), and trypsin (0.25 μg/mL) at 37°C and 5% CO2 until cytopathic effects (CPE) were observed. The cell culture-grown viruses were subjected to 3 cycles of freezing and thawing. Viral RNA was extracted from the supernatant using an RNA extraction kit (QIAGEN, USA), following the manufacturer’s instructions. The extracted RNA was subjected to reverse transcription polymerase chain reaction (RT-PCR) using the primer set CRCoV-SF (5′-GCAATGCTGGTTCGGAAGAG-3′) and CRCoV-SR (5′-GTTGGCATAGGTGAGCACTG-3′) to amplify the CRCoV spike protein [14]. Amplified products were electrophoresed on a 1.5% agarose gel.
Hemagglutination assay
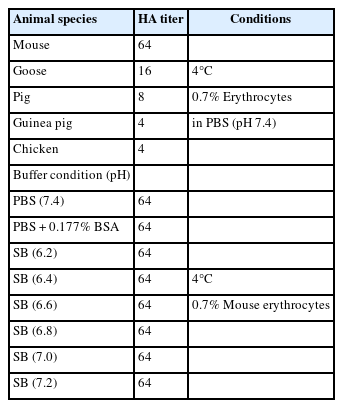
Erythrocytes from mice, geese, chickens, and guinea pigs were collected from the control group of completed clinical study, and pig erythrocytes were obtained from a slaughterhouse. Erythrocytes were washed with phosphate-buffered saline (PBS, pH 7.2) and prepared at a concentration of 0.7% in PBS. The CRCoV supernatant (50 μL, 106 TCID50/mL) was diluted two-fold in U-96-well plates containing PBS. Subsequently, 50 μL of washed erythrocytes were added to each well, and the plates were incubated at 4°C for 1 hour. The hemagglutination assay (HA) titer was determined by identifying the highest dilution of CRCoV that exhibited hemagglutination. To determine the optimal buffer for HA analysis, we evaluated various Sorenson buffers with pH values ranging from 6.2 to 7.2 using the same experimental procedure.
Hemagglutination inhibition test
The HI test for CRCoV was performed as a modified version of the BCoV HI test [15]. Briefly, the dog serum samples, each of 100 μL, were diluted in 500 μL of PBS and mixed with 400 μL of 25% kaolin. After shaking for 1 hour, kaolin was removed by centrifugation at 12,000 rpm for 5 minutes in a microcentrifuge. The resulting clear supernatant was mixed with 50 μL of packed normal mouse erythrocytes. Following a 1 hour incubation at 37°C, the treated serum was separated from the mouse erythrocytes through centrifugation. For the HI test of BCoV or CRCoV, 4 HA units of BCoV or CRCoV (25 μL) were added to 25 μL of the treated sera in U-96-well plates. After incubation for 1 hour at 37°C, 50 μL of 0.7% mouse erythrocytes were added. The plates were then incubated at 4°C for 2 hours. The HI titer was determined as the highest serum dilution that completely inhibited hemagglutination.
Virus neutralization test
The VN test was used to assess the neutralizing activity of antibodies against CRCoV in the serum samples. Forty serum samples were assessed using the VN test to determine their ability to neutralize CRCoV. Briefly, HRT-18 cells were grown to confluency at 37°C with 5% CO2 in a 96-well plate. Serum samples were serially diluted 2-fold in DMEM without fetal bovine serum and mixed with an equal volume of CRCoV (200 TCID50/well). The mixture was incubated at 37°C with 5% CO2 for 1 hour. Following incubation, the mixture was added to the 96-well plate containing HRT-18 cells, along with trypsin (0.25 μg/mL). The presence or absence of CPE induced by the viral infection was observed, and the highest serum dilution that completely prevented CPE was recorded as the VN titer.
Statistical analysis
All statistical analyses were conducted using R statistical software ver. 4.3 (www.cran.org), except for the determination of the 95% confidence interval (CI) of prevalence. The CI for prevalence was calculated using an online program (https://epitools.ausvet.com.au/ciproportion) and the Wilson scoring method. Spearman correlation analysis was performed on log2-transformed data for the HI and VN tests. The receiver operating characteristic (ROC) curve was used to identify the optimal cutoff for the HI test of CRCoV in relation to VN as the gold standard. Chi-square test was used to evaluate differences in seroprevalence based on sex, age, and year of sample collection. Statistical significance was set at p < 0.05. Logistic regression was used to calculate the odds ratios (ORs) for the multivariate risk factors.
Results
Establishment of HI test for CRCoV
CPEs were observed in HRT-18 cells 3 days after CRCoV infection (Fig. 1A and B). CRCoV was also identified by RT-PCR, which amplified the expected size of a spike protein fragment of 442 bp (Fig. 1C). Among the 5 kinds of erythrocytes tested at 4°C, CRCoV exhibited the highest HA activity with mouse erythrocytes, exhibiting 64 HA units (Table 1). No differences were observed between the pH values of the various diluents, and the presence or absence of bovine serum albumin showed no differences. The HI test conditions were determined using mouse erythrocytes and PBS.
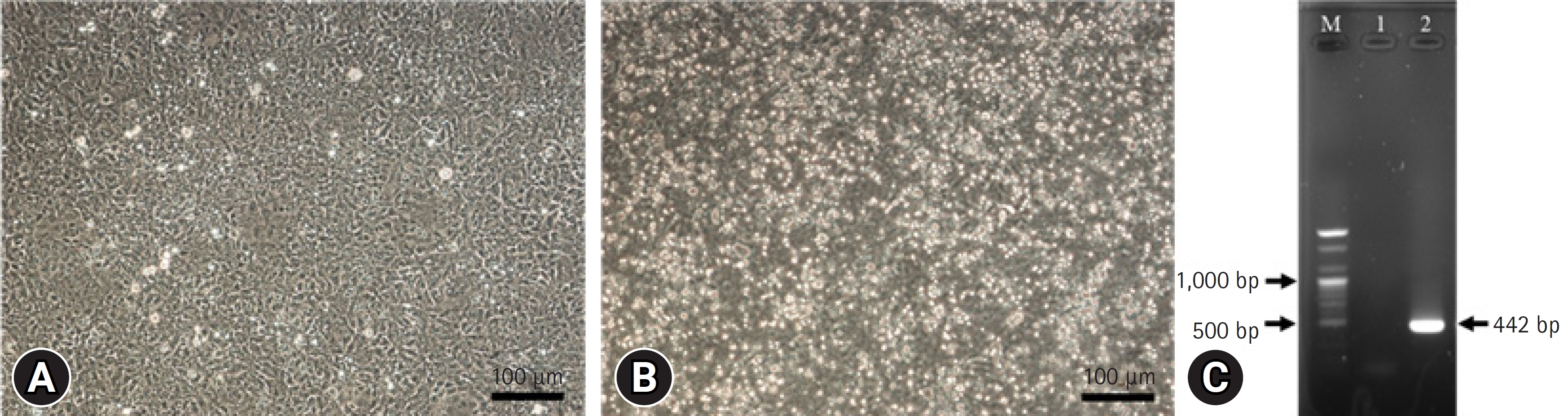
A normal HRT-18 cell (A) and cytopathic effect in HRT-18 cells 3 days after infection of canine respiratory coronavirus (CRCoV), SNU_V22-9 strain (B). Identification of CRCoV using reverse transcription polymerase chain reaction amplification (C). M, 100 bp plus ladder; lane 1, negative control; lane 2, SNU_V22-9 strain (442 bp). Scale bars: (A) 100 μm and (B) 100 μm.
Comparison between VN and HI tests
The VN test was performed using 40 dog serum samples from CRCoV-infected HRT-18 cells to validate and compare HI results using either BCoV or CRCoV antigens. The VN test, considered the “gold standard,” was used to calculate the correlation and sensitivity/specificity/accuracy of HI assays with BCoV and CRCoV antigens. The HI test using the CRCoV antigen showed a strong correlation with VN (R = 0.83), whereas a weaker correlation was observed when the BCoV antigen was used (R = 0.40) (Fig. 2). The optimal cutoff values of the CRCoV antigen for the HI test were estimated using ROC curves. ROC curve analysis was performed to determine the optimal cutoff titer (1:5.7 dilution (Fig. 3). At the optimal cutoff values, the sensitivity and specificity of the HI test were 94.7% and 81.0%, respectively. For practical convenience with two-fold dilutions, a 1:4 dilution, which closely approximated the 1:5.7 dilution, was selected as the final cutoff. At this cutoff, the clinical sensitivity, specificity, and accuracy of the HI test were 90.5%, 78.9%, and 85.0%, respectively (Table 2).
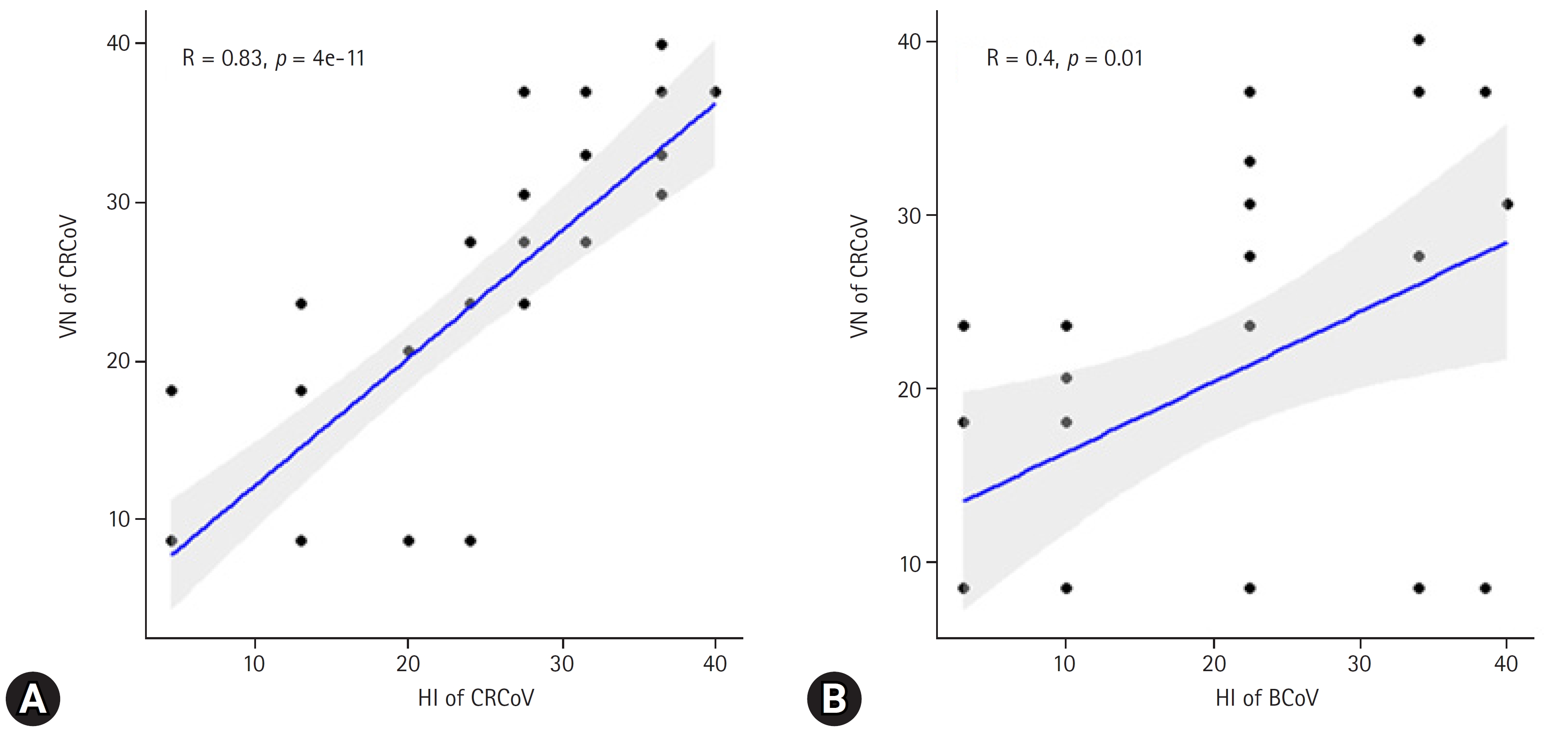
Correlations of hemagglutination inhibition (HI) and virus neutralization (VN) for canine respiratory coronavirus (CRCoV) (A) and bovine coronavirus (BCoV) (B) antigen. The blue lines indicate the correlation between HI and VN titers. The gray–colored area indicates the 95% confidence interval region.
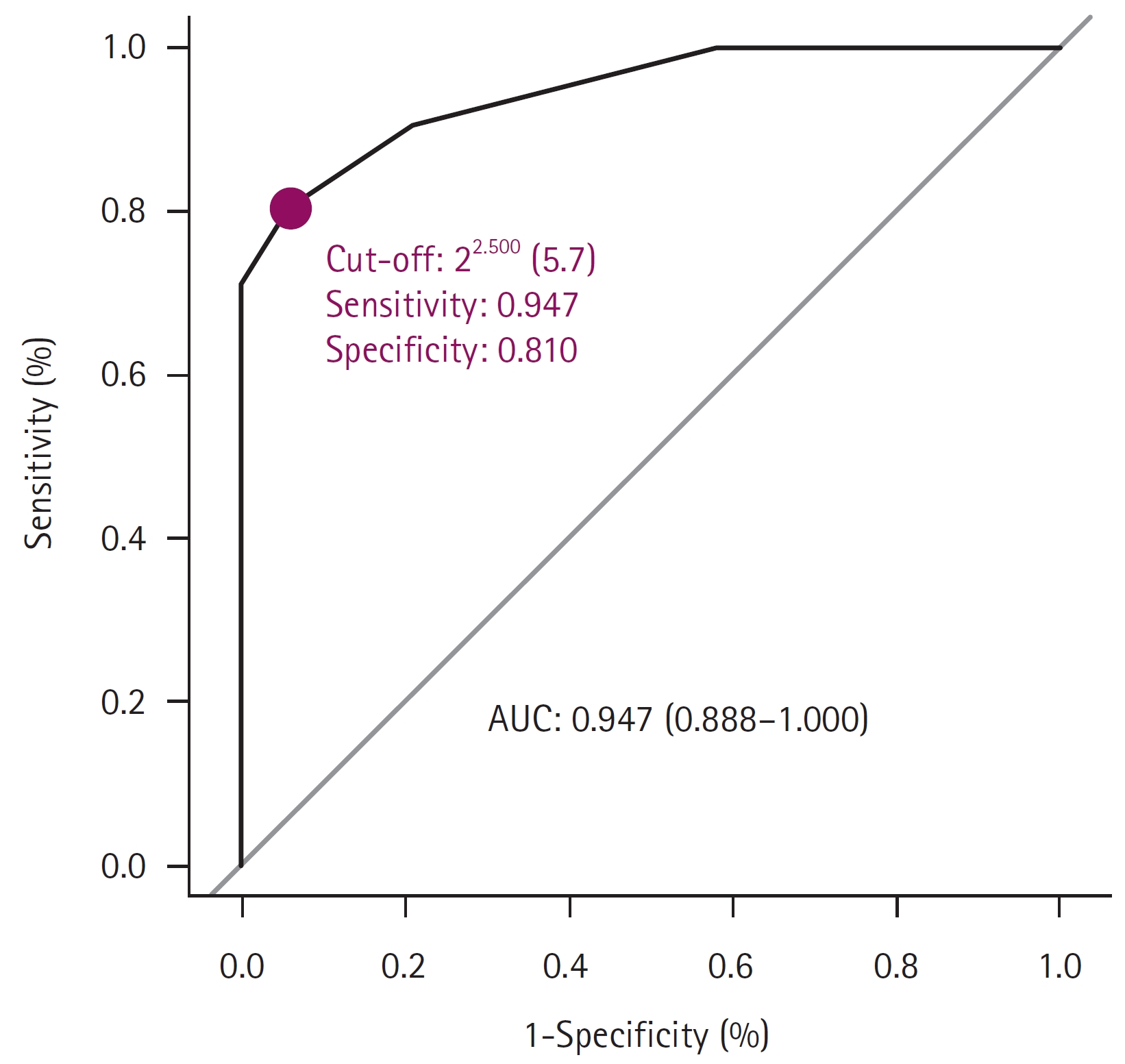
The receiver operating characteristic curves of hemagglutination inhibition and virus neutralization titers for canine respiratory coronavirus. AUC, area under the receiver operating characteristic curve.
Seroprevalence of CRCoV
Between 2019 and 2022, 383 samples were analyzed, with 98, 96, 93, and 96 samples collected in each respective year. The median age of the dogs was 2.1 years, ranging from 12 weeks to 17 years, and 205 of the 383 dogs (53.5%) were male. The overall seropositive rate in South Korea was 200 out of 383 (52.2%; 95% CI, 47.2%–57.1%). The largest proportion of seropositive samples had an HI titer of 1:40 (24.8%), and the number of seropositive samples decreased as the HI titer increased (Fig. 4). The annual seropositive rates were as follows: 59.4% (95% CI, 49.37%–68.65%) in 2022, 53.8% (95% CI, 43.68%–63.55%) in 2021, 30.2% (95% CI, 21.93%–40.01%) in 2020, and 65.3% (95% CI, 55.4%–73.99%) in 2019 (Table 3). The seropositivity for CRCoV in dogs under 1 year of age (43.9%) was similar to dogs classified as 1 to 2 years old (46.7%) (OR, 1.12; 95% CI, 0.66–1.91). Dogs aged 3 to 5 years exhibited a significantly higher positivity rate (66.7%) than dogs aged less than 1 year (OR, 2.54; 95% CI, 1.43–4.59). Male and female dogs had similar seropositivity rates of 52.7% and 51.7%, respectively (p = 0. 93).
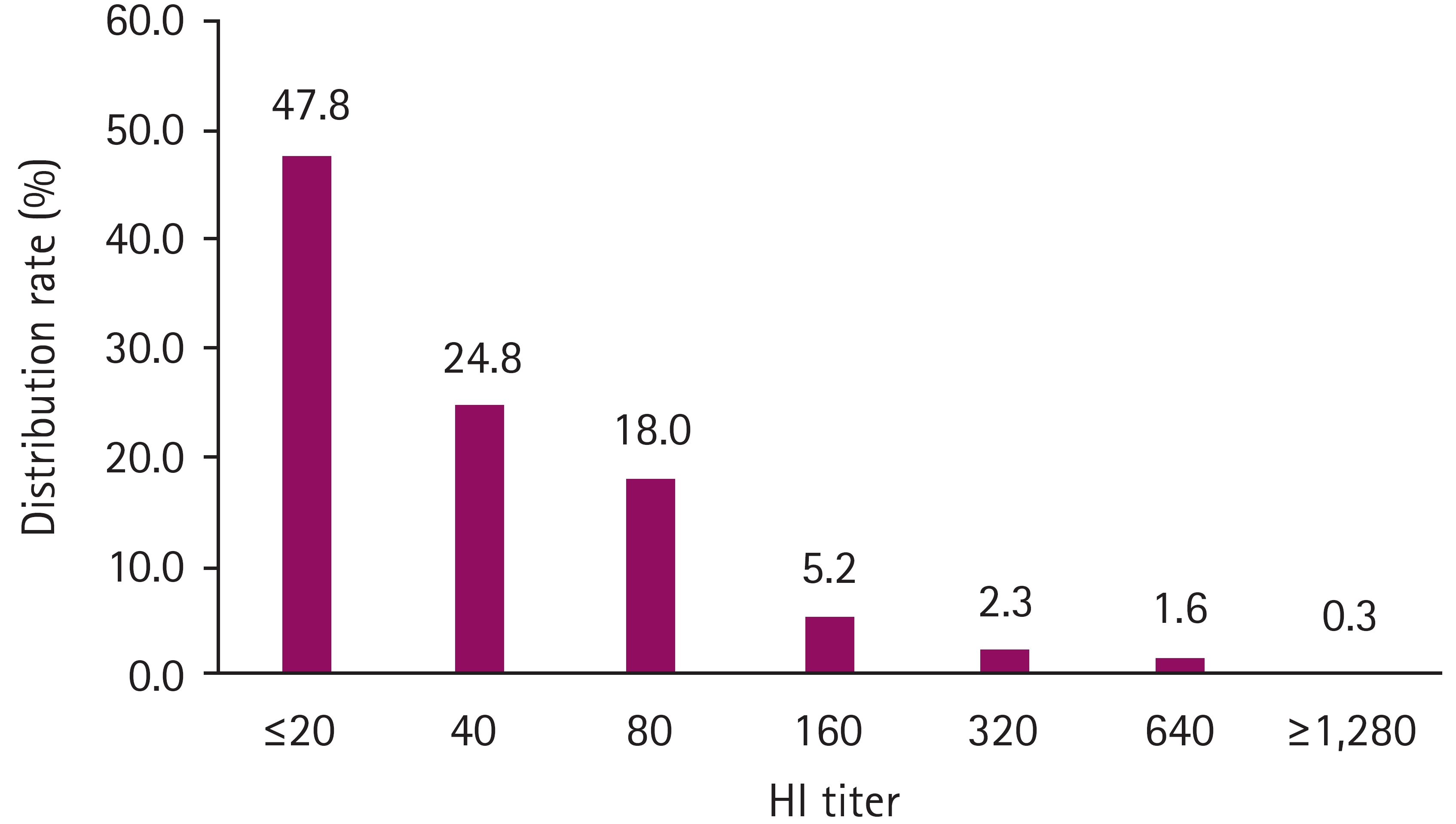
Distribution rate of hemagglutination inhibition (HI) titers against canine respiratory coronavirus (CRCoV) in 383 dogs. Based on the receiver operating characteristic curve analysis, an HI titer of up to 1:40 was considered seropositive against CRCoV.
Discussion
Serological tests against pathogens such as canine parvovirus and Newcastle disease virus have been conducted using the HI test [16,17]. The HI test is cost-effective and can be performed in most laboratories, making it a practical tool for measuring antibodies against pathogens. Our findings show that the HI test for CRCoV exhibits a strong correlation (R = 0.83, p < 0.001) with the VN test and an area under the curve of 0.947 in the ROC analysis. The sensitivity and specificity of the BCoV-based HI test using dog sera, when compared to BCoV-ELISA, were 23.8% and 85.1%, respectively [13]. In our study, the BCoV antigen-based HI test exhibited a weaker correlation than the VN test (R = 0.4). This result indicates that the HI test for detecting CRCoV antibodies demonstrated low cross-reactivity with BCoV antigens. Considering the possibility of BCoV infections in dogs and the reports of increased levels of BCoV-neutralizing antibodies in dogs infected with BCoV [18], the CRCoV antigen-based HI test provides a precise and cost-effective means of monitoring CRCoV infections in dog populations.
CRCoV is widely distributed across North America, Europe, and Asia, with reported seroprevalence rates of 59.1%, 54.7%, 36%, and 17.8% in Canada, the United States, the United Kingdom, and Japan, respectively [8,19]. Additionally, studies conducted in the United States and the United Kingdom have revealed regional variations in CRCoV seroprevalence, with higher rates observed in densely populated urban areas [8]. In the present study, we confirmed an increase in CRCoV circulation and exposure in dogs in Korea. Overall, 52.2% of dog serum samples tested positive for CRCoV antibodies, signifying a substantial increase when compared to the reported seropositivity rate of 12.8% in South Korea in 2010 [9]. The increased exposure to CRCoV in South Korea may be attributed to dog-to-dog contact at dog shelters and veterinary clinics. For a more comprehensive understanding, future research should expand their investigation to encompass additional regions of South Korea.
CRCoV seroprevalence varies with age. Age-related seropositivity in dogs may be attributed to an increased likelihood of continuous viral exposure as the dogs age. Conversely, dogs over 6 years of age show moderate seroprevalence, which could be due to factors such as reduced activity levels and a potential decline in immune response efficiency [20]. Similar age-related patterns were observed in studies conducted in other countries [8]. In the United States and the United Kingdom, age influences the seroprevalence of CRCoV antibodies, with dogs under 1 year of age having a higher likelihood of being seronegative for CRCoV than older dogs (p < 0.001) [8]. In Italy, the percentage of dogs with CRCoV antibodies increases with age, reaching a peak seroprevalence of 40.0% (12/30) in 9-year-old dogs and subsequently declining [20]. In this study, the lowest seroprevalence of CRCoV was observed in dogs under 1 year of age, similar to findings in the United States and the United Kingdom. Conversely, the highest seroprevalence was identified in dogs between 3 and 5 years of age, akin to the pattern observed in Italy, and decreased among older dogs. This indicates that the seroprevalence of CRCoV in dogs in Korea varies with age.
In conclusion, the HI test has exhibited high specificity with the VN test of CRCoV. Among the study population, 52.2% of dogs in Korea were seropositive, which is comparable to rates reported in the United States. These findings imply that dogs in Korea are continuously exposed to CRCoV. In the future, the development of CRCoV-specific ELISA and vaccines will be essential to prevent CRCoV infections. This information will contribute to a better understanding of the epidemiology and distribution of CRCoV and may prove invaluable for the effective prevention of CRCoV.
Notes
The authors declare no conflict of interest.
Acknowledgements
The authors are grateful to Dr. Dea-Sub Song of Seoul National University for depositing canine respiratory coronavirus SNU_V22-9 strain to Korea veterinary culture collection.
This work was supported financially by a grant (N-AD20-2010-19-01) from the Animal, and Plant Quarantine Agency, Ministry of Agriculture, Food and Rural Affairs, Republic of Korea.