Serological responses and protection levels in chickens administered with Newcastle disease vaccines
Article information
Abstract
Vaccination against Newcastle disease (ND) is the most effective means of controlling the disease, and these vaccines are commercialized only after their safety and effectiveness have been verified through tests that comply with Korean Standards of National Lot Release for Veterinary Biologics. This study investigated whether a relatively convenient and safe serological test can be used in place of the challenge test using highly virulent ND virus. Hemagglutination inhibition (HI) assay and enzyme-linked immunosorbent assay (ELISA) were considered positive of log2 2 or more and cutoff value of 200 or more, respectively, in both live and inactivated vaccines. However, when the antibody levels of the live and inactivated vaccines induced using the Ulster 2C, KBNP-C4152R2L, and K148/08 strains were compared, the antibody titers for inactivated vaccines were significantly higher than those for live vaccines in both the HI assay and ELISA. A strong positive correlation was observed between HI and ELISA antibody titers. The live vaccines corresponded to a survival rates of ≥ 80% and the inactivated vaccines corresponded to 100% survival rates. This study confirmed that standard efficacy tests can serve as serological tests, and can replace the challenge test and that the vaccine approval process can be improved.
Introduction
Newcastle disease (ND) is one of the most critical infectious diseases in domestic and wild birds [1]. Generally, ND primarily causes acute respiratory disease; however, it can also lead to depression, nervous manifestations, and diarrhea [2]. ND virus (NDV) or Avian paramyxoviruses 1 (APMV-1) was recently classified to Family Paramyxoviridae, Genus Avian Orthoavulavirus and common NDV is recently known as Avian Orthoavulavirus-1 (AOAV-1) [3]. Although only a single serotype of NDV has been found to be exist as determined using neutralizing tests and cross-protective analysis [4,5], the virus can be categorized into 2 major classes based on phylogenetic analysis of the fusion (F) gene sequence, i.e., Classes I and II [3]. Moreover, Kapczynski et al. [6] reported that NDV is constantly evolving and that it can be classified into more than 21 phylogenetically distinct genotypes as per epidemiological evidence. Class I NDVs are genetically less diverse, are present in wild waterfowl, and have low virulence; in contrast, Class II NDVs are more diverse, frequently isolated from poultry with occasional spillovers into wild birds, and have a wide range of virulence. Moreover, Class II NDV can be subdivided into 21 genotypes [1,4,7].
Generally, virulent NDV can be defined as NDV with an intracerebral pathogenicity index of ≥ 0.7 in 1-day-old chicks or multiple basic amino acids at the cleavage site of the virus F protein, according to the World Organisation for Animal Health (OIE) [8]. NDV isolates can be classified into 3 major pathological types based on virulence: velogenic (high virulence), mesogenic (moderate virulence), and lentogenic (low virulence) [9]. Velogenic NDV can be subdivided into viscerotropic velogenic NDV (vvNDV), which causes severe visceral and intestinal hemorrhage, and neurotropic velogenic NDV, which presents with severe neurologic clinical signs, depending on the clinical signs in chicken behavior [10]. ND is one of the damaging diseases among chickens, and it has a significant global economic impact worldwide [11]. Moreover, there are no reports of treatment of ND and no antiviral drugs are commercially available.
Vaccination against ND is the most effective means of controlling the disease, and this method is practiced in most countries producing commercial and backyard poultry except Ireland, Norway, Switzerland or Sweden [12–14]. In Korea, vaccination against ND has been mandatory in hatcheries and poultry farms since 2001; consequently, no ND outbreaks have occurred since 2010 [15]. However, ND vaccination is still being used for prevention of outbreaks in the future. These vaccines are commercialized only after their safety and effectiveness have been verified through tests that comply with Korean Standards of National Lot Release for Veterinary Biologics [16]. Although vaccine validation is currently performed as a challenge test in chickens using the highly virulent NDV, this study investigated whether a relatively convenient and safe serological test can be used in place of the challenge test.
Materials and Methods
Experimental animals and NDV vaccine
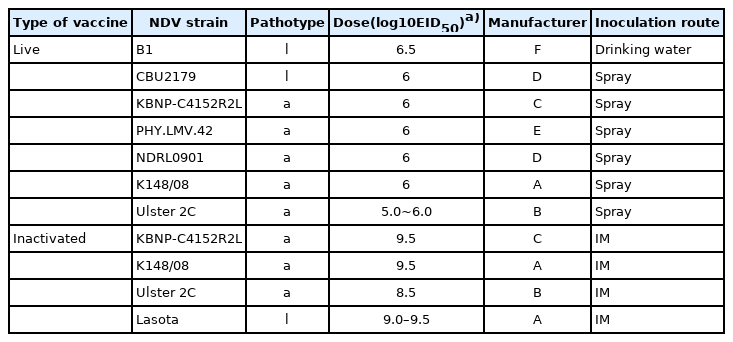
Overall, 11 NDV vaccines from 6 manufacturers were tested (Table 1). The age of the test animals and the dose and inoculation route of the vaccines for evaluating vaccine strains were in accordance with the Korean Standards of National Lot Release for Veterinary Biologics [16] and the manufacturers’ recommendations. Namely, 6 vaccine strains among 7 live vaccine strains were inoculated by spray route using an automatic spray device (SKmos2009; Samkwang, Korea) in each of the 25 1-day-old specific-pathogen-free (SPF) chickens (Namduck SPF, Korea). The other live vaccine strains were inoculated by orally using a 1-mL syringe in 10 3-weeks-old and 10 6-weeks-old SPF chickens, respectively. Four inactivated vaccine strains were intramuscularly injected into 25 6-weeks-old SPF chickens. All experiments were performed thrice, and the mean outcomes were considered. The vaccinated and control groups were placed in separate isolation units throughout the study. All procedures in this study were conducted in accordance with the Institutional Animal Care and Use Committee (IACUC), APQA (approval ID: 2019-452).
Serum collection
To detect NDV antibodies, blood samples from the different groups administrated with the live (n = 10/group) and inactivated (n = 10/group) NDV vaccines were collected at 2 and 3 weeks after immunization, respectively, based on Korean Standards of National Lot Release for Veterinary Biologics [16]. Serum was separated and subjected to heat-induced inactivation at 56°C for 30 min. The samples were stored at −20°C until further analysis.
Challenge experiment
The vvNDV strain Kr005 was used as the challenge virus in this study [17]. To determine the concentration of Kr005, 50% egg infectious dose (EID50) was measured using the Reed-Muench method [18]. Virus challenge involved intramuscular injection of Kr005 (1 × 105 EID50/0.5 mL) at 2 and 3 weeks after the administration of live and inactivated vaccines, respectively. The challenged chickens were observed daily for clinical signs, mortality for 2 weeks.
Hemagglutination inhibition assays
Hemagglutination inhibition (HI) assay was performed using U-bottomed 96-well microtiter plates as recommended by the OIE manual of standard diagnostic tests [8]. The assay was performed using 4 hemagglutination units of the NDV LaSota antigen. The endpoint was the reciprocal of the highest serum dilution that completely inhibited hemagglutination. HI titers were also expressed as log2.
Enzyme-linked immunosorbent assay
Indirect enzyme-linked immunosorbent assay (ELISA) was conducted to detect NDV antibodies in the serum samples using a commercial ELISA kit (MEDIAN Diagnostics Inc., Korea) according to the manufacturer’s instructions. An absorbent 450-nm filter was used to calculate the sample-to-positive (S/P) ratio of each sample using an ELISA microplate reader (Molecular Devices, USA). The presence or absence of antibodies against to NDV was determined by correlating the absorbance value of the sample to the positive control mean, and positive was indicated by an S/P ratio of > 0.2. The ELISA titer (log titer) was calculated as 1.8 (log S/P) + 3.56.
Statistical analyses
GraphPad Prism ver. 9.0 (GraphPad Software Inc., USA) was used for all statistical analyses. To compare the serological response and analyze the relationship between the antibody titers and the survival rate, independent-sample t-test and Pearson two-tailed analysis were performed. A p-value of < 0.0001 was considered statistically significant.
Results
Serological response the SPF chickens to NDV vaccine
The serological response of the chickens to the vaccine strains is presented in Fig. 1. The HI assay results revealed that the mean antibody titers against the live vaccine strains ranged from log2 4.7 ± 1.6 to log2 6.4 ± 1.7, whereas those against the inactivated vaccine strains ranged from log2 8.7 ± 1.0 to log2 9.7 ± 0.8. For both live and inactivated vaccines, antibody titers were considered positive of log2 2 or more. ELISA revealed that the mean antibody titers against the live and inactivated vaccine strains ranged from 3,655 ± 3,741 to 6,601 ± 2,297 and from 8,860 ± 3,101 to 12,338 ± 2,016, respectively. All values exceeded the manufacturer’s positive cutoff value of 200. However, when the antibody levels of the live and inactivated vaccines induced using the Ulster 2C, KBNP-C4152R2L, and K148/08 strains were compared, the antibody titers for inactivated vaccines were significantly higher than those for live vaccines in both the HI assay and ELISA (p < 0.0001).
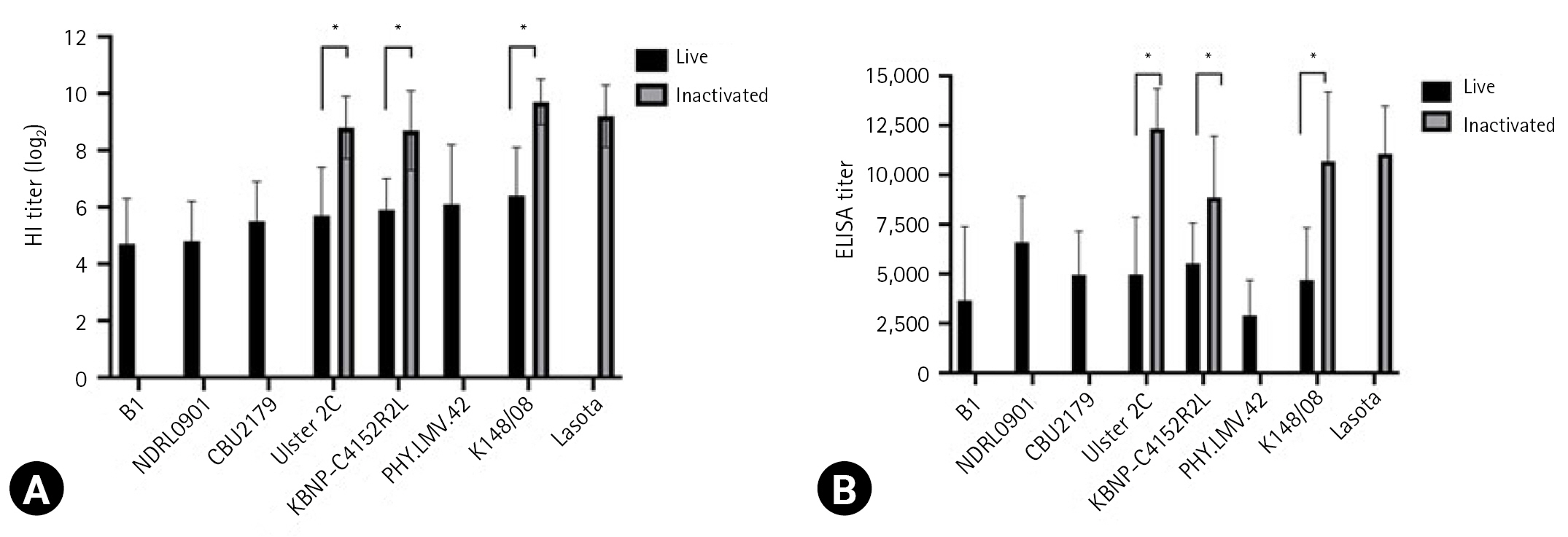
Serological responses between chickens vaccinated with 2 Newcastle disease virus (NDV) groups, live and inactivated NDV vaccines. Hemagglutination inhibition (HI) titers (A) and enzyme-linked immunosorbent assay (ELISA) titers (B) were determined as the means antibody value and ± standard deviation (SD). Significant differences of antibody levels between live and inactivated vaccines are indicated by asterisk (p < 0.0001). Data represent the mean ± SD of 3 independent experiments.
Correlation between HI and ELISA titers
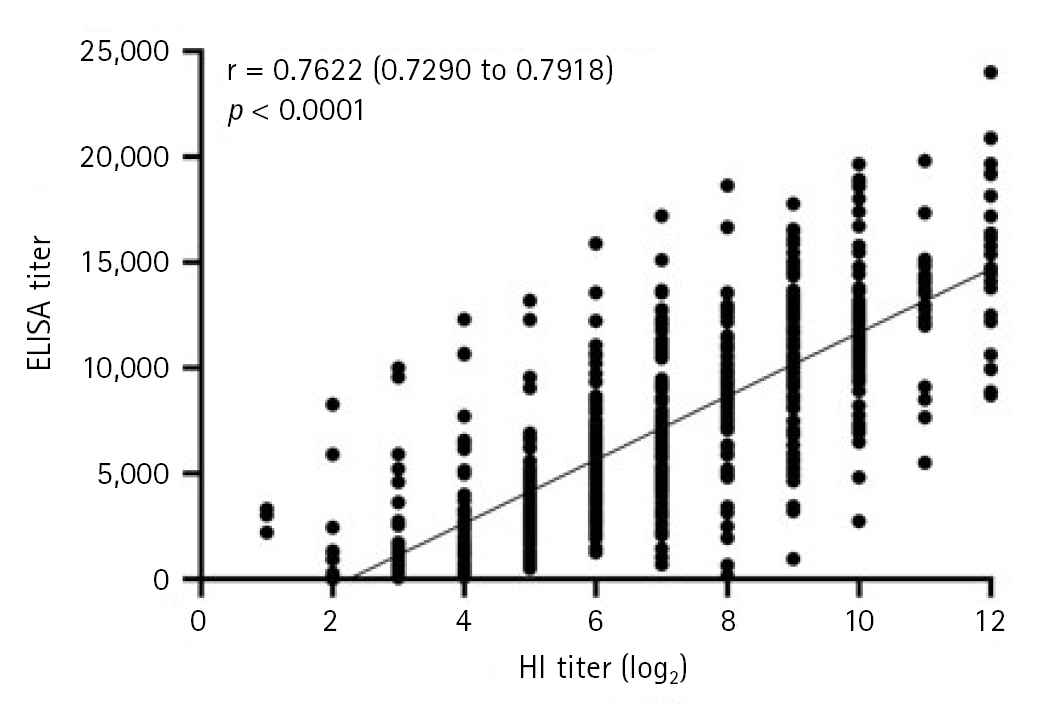
The correlation between HI and ELISA antibody titers is presented in Fig. 2. A strong positive correlation was observed between HI and ELISA antibody titers (Pearson correlation coefficient r, 0.7622; 95% confidence interval, 0.7290–0.7918; p < 0.0001).
Relationship between serological responses and protection levels
The relationship between antibody titers and survival rates is presented in Fig. 3. According to Korean Standards of National Lot Release for Veterinary Biologics, a vaccine is considered effective when the survival rates after vaccination with live and inactivated vaccines are ≥ 80 and ≥ 90%, respectively [16]. The mean HI and ELISA titers for live vaccines ranged from log2 3.1 to log2 9 and 2,316 to 11,965, respectively, corresponding to a survival rates of ≥ 80%. Further, the mean HI and ELISA titers for inactivated vaccines ranged from log2 7 to log2 11.4 and 6,808 to 16,838, respectively, both of which corresponding to 100% survival rates.
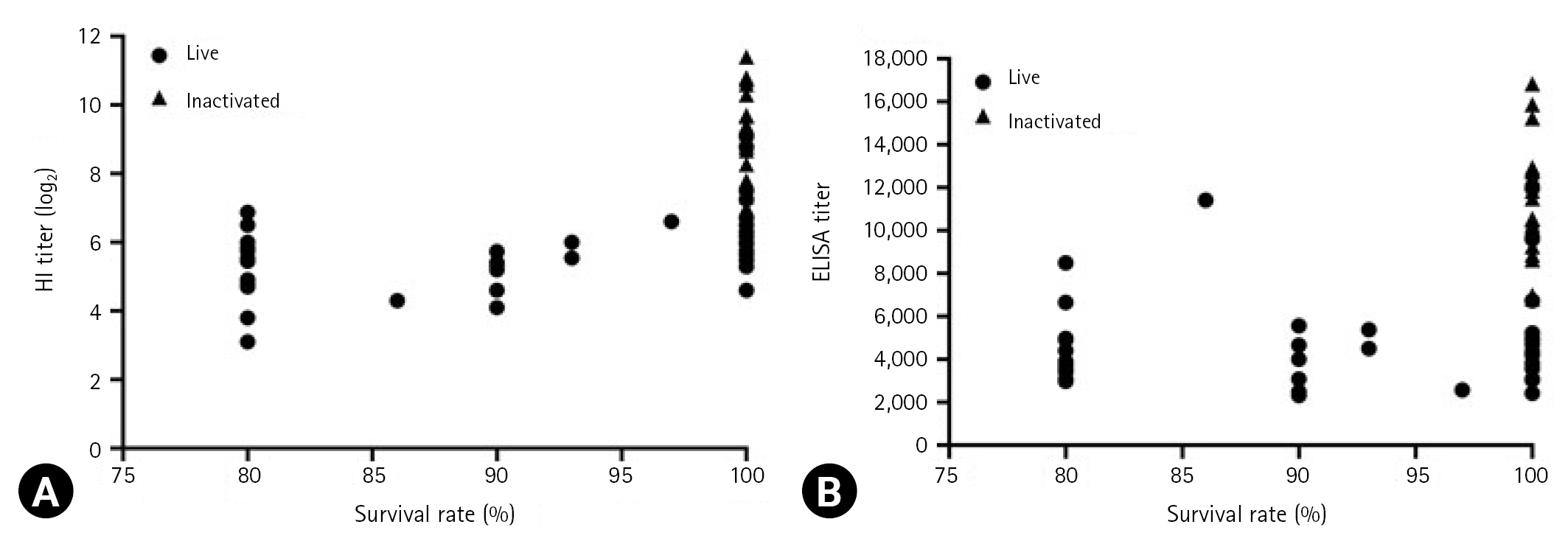
Relationship between antibody titers and survival rate (%). Hemagglutination inhibition (HI) titers (A) and enzyme-linked immunosorbent assay (ELISA) titers (B) were determined, and the survival rates of live and inactivated were indicated, respectively. Data represent the mean ± standard deviation of 3 independent experiments.
Discussion
All NDVs are of the same serotype and therefore, have similar antigenic properties; this results in a uniform immune response regardless of the vaccine strain. Both live and inactivated NDV vaccines have been widely used globally to contain the economic threat posed by ND outbreaks [6,19]. In Korea, national efficacy trials have been conducted to ensure that vaccines against ND are safe and effective in generating a protective immune response in chickens [16]. To safely manage vvNDV as a challenge virus, biosafety level 3 facilities must be developed and utilized by 2023; however, in reality, this is difficult because of problems of cost and lack of facilities in Korea [20]. Therefore, this study investigated whether the efficacy of the vaccines could be evaluated using a more convenient and safe serological test than the challenge test.
In this study, all 11 vaccines, including 7 live vaccines and 4 inactivated vaccines, achieved the positive criterion of values of log2 2 or higher in the HI assay and values exceeding the positive cutoff value of 200 in ELISA. Moreover, the HI and ELISA antibody titers generated by the inactivated vaccines were significantly higher than those generated by the live vaccines. Inactivated vaccines are generally produced as oil adjuvants to enable stimulation for a relatively long time, and they tend to increase the level of humoral antibodies in the blood after intramuscular administration [21]. Moreover, adjuvants can effectively stimulate immunity by inducing antibody production, and high antibody titers can protect against morbidity or mortality associated with NDV infection [21].
In contrast, lentogenic NDV strains are widely used as live vaccines in young birds aged 1–14 days; these vaccines block infection by inducing early immunity through cellular, local mucosal, and humoral immune responses [12]. The biggest advantage of live vaccines is that they can be administered through various routes, such as through drinking water, spray, or eye drops, and are suitable for mass distribution, resulting in extremely low costs from production to administration [6,12]. NDV comprises an envelope containing the hemagglutinin–neuraminidase (HN) protein that allows the virus to bind to host cells and the F glycoprotein that promotes the fusion of the envelope to host cells [6]. Antibodies against HN play a role in blocking virus attachment, and antibodies against the F glycoprotein inhibit virus fusion with host cell membranes, making them the primary targets for immune responses [22,23]. In this study, these antibody titers of the ND vaccine strains displayed different trends in the HI assay and ELISA, as previously described [24,25]. This is because the HI assay only detects antibodies against the HN protein, whereas the ELISA uses the whole virus as antigen, thus potentially allowing the detection of antibodies against any protein on the NDV particle [26,27]. Moreover, Thayer et al. [28] reported that ELISA could detect antibodies that did not act as protective antibodies or less analyze the role of HN in antibody induction.
However, it is impossible to unequivocally state whether this significant difference is attributable to strain characteristics. This is because although commercial vaccines contain verified vaccine strains, the composition differs. In addition, chickens vaccinated against ND produce IgM, IgY, and IgA antibodies as part of the immune response [29]. Antibodies are detected in blood 6 days after inoculation, reaching peak levels at 3 to 4 weeks resulting in high titers [30,31].
In this study, the results of the HI assay and a ready-made ELISA displayed a strong positive correlation, as previously described [32]. However, Marquardt et al. [33] reported no correlation between HI assay and ELISA. The results in this study also found that the number of positive samples was higher in the HI assay than in ELISA. In total, 0.4% of HI-negative sera samples (HI titer < log2 2) were positive on ELISA, whereas 1.7% of HI-positive sera samples were negative on ELISA (ELISA titer < 200). The results of HI assay differed from those of ELISA, and it is different from what is known that the specificity of ELISA is higher than the HI assay [32]. This discrepancy between the 2 assays may be related to the aforementioned sensitivity characteristics. Moreover, because commercial ELISA kits have different antigens for each product, differences in sensitivity and specificity between kits must also be considered. Chickens vaccinated using live and inactivated vaccines exhibited survival rates of > 80% and 100%, respectively. Thus, this study found a strong correlation between protection against challenge infection and serological titers, which is consistent with the results of previous studies [34–36]. This study confirmed that standard efficacy tests can serve as serological tests, and can replace the challenge test and that the vaccine approval process can be improved through shortening the trial periods, considering animal welfare concerns, and reducing costs.
Notes
The authors declare no conflict of interest.
Acknowledgements
This research was supported by a grant from the Animal and Plant Quarantine Agency of the Republic of Korea (no. B-1543073-2021-23-01).