1. Di Martino B, Marsilio F, Roy P. Assembly of feline calicivirus-like particle and its immunogenicity. Vet Microbiol 2007;120:173-178.


2. Yumiketa Y, Narita T, Inoue Y, Sato G, Kamitani W, Oka T, Katayama K, Sakaguchi T, Tohya Y. Nonstructural protein p39 of feline calicivirus suppresses host innate immune response by preventing IRF-3 activation. Vet Microbiol 2016;185:62-67.


5. Radford AD, Addie D, BelĂĄk S, Boucraut-Baralon C, Egberink H, Frymus T, Gruffydd-Jones T, Hartmann K, Hosie MJ, Lloret A, Lutz H, Marsilio F, Pennisi MG, Thiry E, Truyen U, Horzinek MC. Feline calicivirus infection. ABCD guidelines on prevention and management. J Feline Med Surg 2009;11:556-564.


6. Zhao Y, Chen X, Ying Y, Wang K, Dong H, Gao C, Yang S, Hu G. Isolation and phylogenetic analysis of three feline calicivirus strains from domestic cats in Jilin Province, China. Arch Virol 2017;162:2579-2589.


7. Tian J, Liu D, Liu Y, Wu H, Jiang Y, Zu S, Liu C, Sun X, Liu J, Qu L. Molecular characterization of a feline calicivirus isolated from tiger and its pathogenesis in cats. Vet Microbiol 2016;192:110-117.


8. Gabriel SS, Tohya Y, Mochizuki M. Isolation of a calicivirus antigenically related to feline caliciviruses from feces of a dog with diarrhea. J Vet Med Sci 1996;58:1041-1043.


9. Coyne KP, Jones BR, Kipar A, Chantrey J, Porter CJ, Barber PJ, Dawson S, Gaskell RM, Radford AD. Lethal outbreak of disease associated with feline calicivirus infection in cats. Vet Rec 2006;158:544-550.


10. Radford AD, Coyne KP, Dawson S, Porter CJ, Gaskell RM. Feline calicivirus. Vet Res 2007;38:319-335.


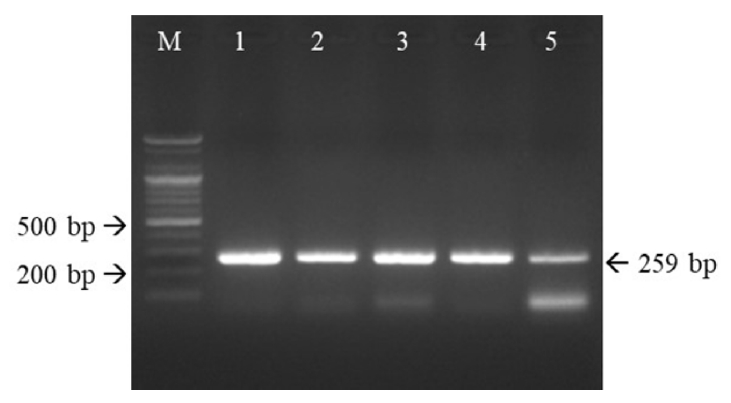
11. Marsilio F, Di Martino B, Decaro N, Buonavoglia C. A novel nested PCR for the diagnosis of calicivirus infections in the cat. Vet Microbiol 2005;105:1-7.


12. Litster A, Wu CC, Leutenegger CM. Detection of feline upper respiratory tract disease pathogens using a commercially available real-time PCR test. Vet J 2015;206:149-153.


13. Jung JY, Lee K, Choi EJ, Lee H, Moon BY, Kim HY, So B. Cat diseases diagnosed in Korea, 2015~2017. Korean J Vet Serv 2018;41:119-123.
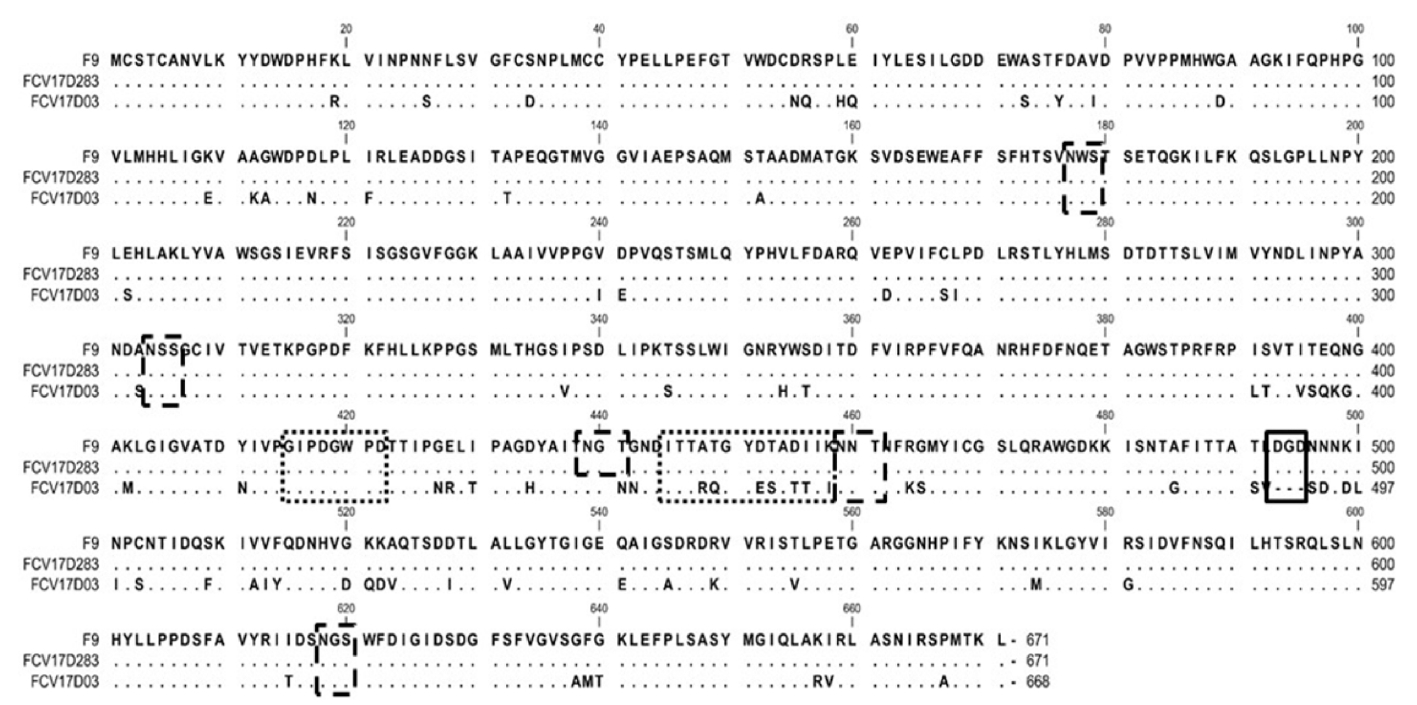
14. Guo H, Miao Q, Zhu J, Yang Z, Liu G. Isolation and molecular characterization of a virulent systemic feline calicivirus isolated in China. Infect Genet Evol 2018;65:425-429.


16. Caringella F, Elia G, Decaro N, Martella V, Lanave G, Varello K, Catella C, Diakoudi G, Carelli G, Colaianni ML, Bo S, Buonavoglia C. Feline calicivirus infection in cats with virulent systemic disease, Italy. Res Vet Sci 2019;124:46-51.


18. Prikhodko VG, Sandoval-Jaime C, Abente EJ, Bok K, Parra GI, Rogozin IB, Ostlund EN, Green KY, Sosnovtsev SV. Genetic characterization of feline calicivirus strains associated with varying disease manifestations during an outbreak season in Missouri (1995-1996). Virus Genes 2014;48:96-110.


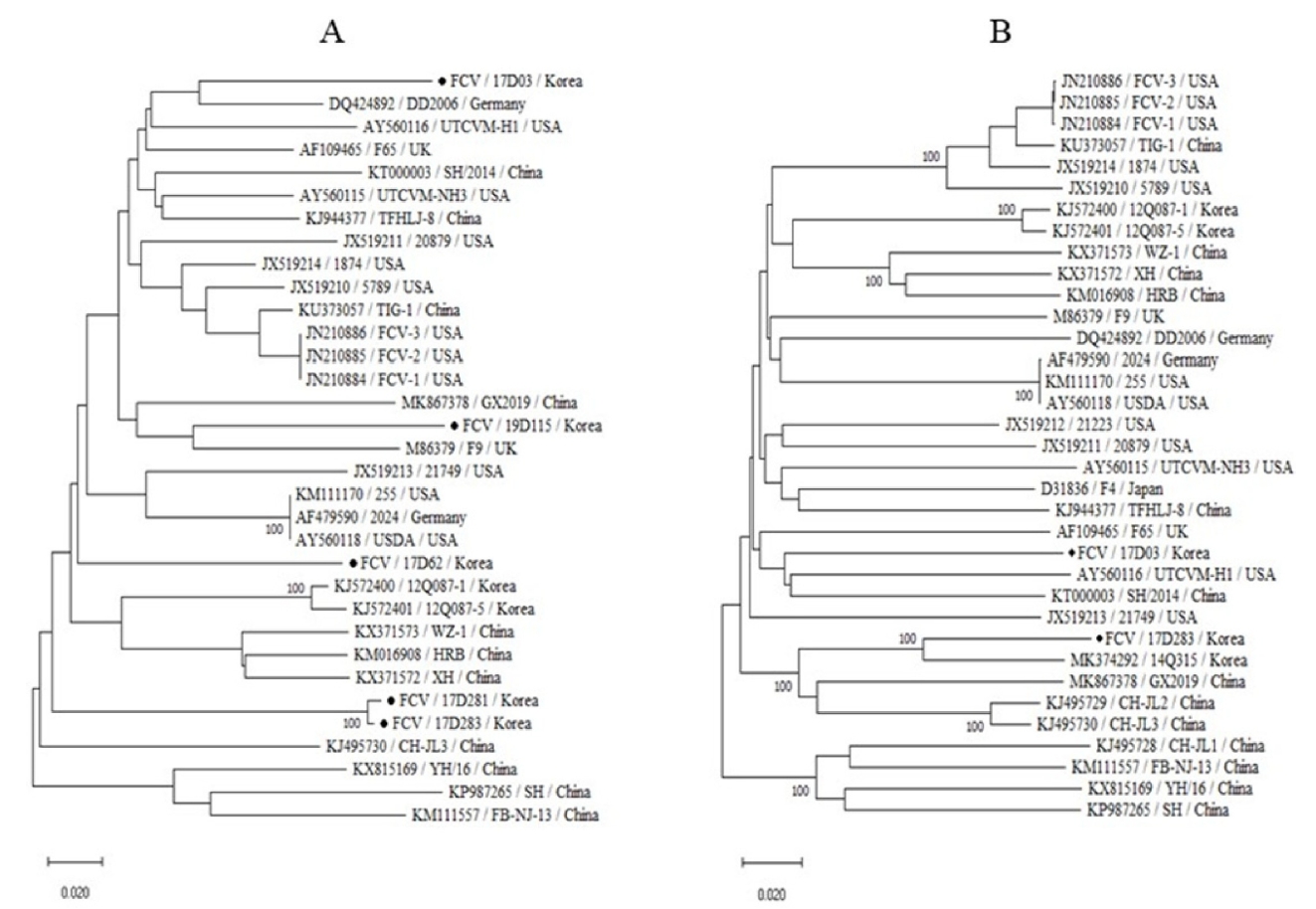
19. Sun Y, Deng M, Peng Z, Hu R, Chen H, Wu B. Genetic and phylogenetic analysis of feline calicivirus isolates in China. Vet J 2017;220:24-27.


20. Glotova TI, Semenova OV, Nikonova AA, Glotov AG, Vyatkin YV, Bondar AA. [Isolation and phylogenetic analysis of feline calicivirus in Siberia]. Vopr Virusol 2018;63:268-274.


21. Masubuchi K, Wakatsuki A, Iwamoto K, Takahashi T, Kokubu T, Shimizu M. Immunological and genetic characterization of feline caliciviruses used in the development of a new trivalent inactivated vaccine in Japan. J Vet Med Sci 2010;72:1189-1194.


22. Brunet S, Sigoillot-Claude C, Pialot D, Poulet H. Multiple correspondence analysis on amino acid properties within the variable region of the capsid protein shows differences between classical and virulent systemic feline calicivirus strains. Viruses 2019;11:1190.






















 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



